Search


USFDA Med Dev Guidance: Animal Studies for Dental Bone Grafting Material Devices in 510(k) Submissions
On 22 August 2025 , the U.S. Food and Drug Administration (FDA) released a comprehensive guidance document titled “ Animal Studies for...

Sharan Murugan
Aug 23, 20252 min read


USFDA MD Guidance: Enhancing Safety and Efficiency in Orthopedic Devices and Sterilization Processes
The USFDA Center for Devices and Radiological Health has recently issued three key final guidance documents focusing on orthopedic...

Sharan Murugan
Nov 27, 20242 min read

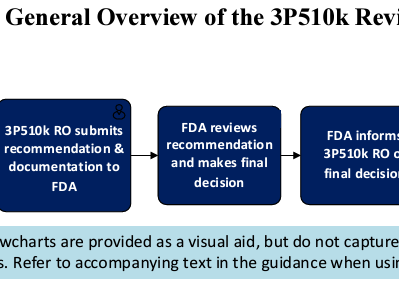
USFDA Guidance: 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review
The 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review are pivotal initiatives by the USFDA to...

Sharan Murugan
Nov 20, 20242 min read


USFDA Guidance: Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions
Yesterday (08 January, 2024) the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research Center for Devices...

Sharan Murugan
Jan 9, 20241 min read
![USFDA Guidance: Premarket Notification [510(k)] Submissions](https://static.wixstatic.com/media/nsplsh_453363554d4e4263424363~mv2.jpg/v1/fill/w_333,h_250,fp_0.50_0.50,q_30,blur_30,enc_avif,quality_auto/nsplsh_453363554d4e4263424363~mv2.webp)
![USFDA Guidance: Premarket Notification [510(k)] Submissions](https://static.wixstatic.com/media/nsplsh_453363554d4e4263424363~mv2.jpg/v1/fill/w_399,h_300,fp_0.50_0.50,q_90,enc_avif,quality_auto/nsplsh_453363554d4e4263424363~mv2.webp)
USFDA Guidance: Premarket Notification [510(k)] Submissions
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health and Center for Biologics Evaluation and Research...

Sharan Murugan
Sep 10, 20232 min read


USFDA Med. Device: Guidance on Laser Products, Diagnostics, X-ray & Ultrasonic Diathermy Devices
The Center for Devices and Radiological Health released multiple guidances earlier today (21-February-2023), related to "Laser Products,...

Sharan Murugan
Feb 21, 20232 min read


USFDA’s Electronic Submission Template for Medical Device 510(k) Submissions
On 28-September, 2021 USFDA released draft guidance to assist sponsors in using an electronic template for submitting a premarket...

Sharan Murugan
Sep 29, 20211 min read


Draft MD Guidance on Performance Criteria for Safety and Performance Based Pathway - USFDA
On 30th August 2021, the Center for Devices and Radiological Health released 2 new draft guidance related to Premarket, 510(k). i.e.,...

Sharan Murugan
Aug 30, 20211 min read
