Search


USFDA Guidance: Use of Artificial Intelligence To Support Regulatory Decision & Guiding Principles of Good AI Practice in Drug Development
Artificial Intelligence (AI) is no longer a future concept in pharmaceutical research—it is already influencing how drugs and biological products are discovered, developed, manufactured, and monitored throughout their lifecycle. However, when AI outputs are used to support regulatory decisions , questions of trust, transparency, and accountability become critical. Recognising this, global regulators have begun defining expectations for the responsible use of AI in drug devel
Sharan Murugan
Jan 313 min read


Regulatory Intelligence Sources and Databases: A Complete Guide for Today’s Pharma, Biotech, and MedTech Teams
Staying compliant in the life sciences industry has never been more complex. Regulatory changes move faster than ever, global markets evolve continuously, and expectations from agencies keep rising. In this environment, regulatory intelligence is no longer a luxury. It is a core operational pillar. For my 1000th post on Regulatory Affairs News, I’m sharing a full breakdown of the latest regulatory intelligence sources, tools, databases, and best practices . I’m also attachin
Sharan Murugan
Nov 8, 20252 min read

European Commission: Guidelines on the Scope of the Obligations for General-Purpose AI Models established by AI Act
On 18 July 2025, the European Commission published detailed Guidelines on the scope of the obligations for providers of general-purpose...
Sharan Murugan
Jul 19, 20253 min read

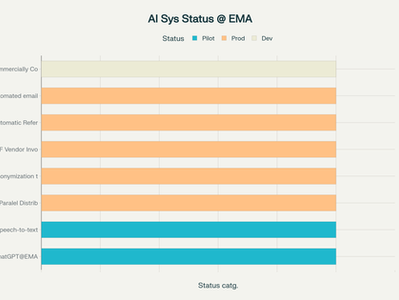
EMA’s AI Journey: The Rise of Artificial Intelligence in Medicines Regulation
In a world where artificial intelligence (AI) is moving rapidly from theory to practice, regulators are tasked with keeping pace—not only...
Sharan Murugan
Jul 19, 20253 min read


Meet ELSA: USFDA Launches Agency-Wide AI Tool to Optimize Performance
What if the agonizing wait for drug approvals could be slashed from days to minutes? Imagine a future where breakthrough therapies reach...
Sharan Murugan
Jun 2, 20252 min read
