Search


USFDA & ICH Guidances: : cGMP for Medical Gas, Extractables & Leachables (Q3E), and Class 3 Leachable Monographs
As pharmaceutical modalities diversify—from traditional injectables to complex biologics, cell therapies, inhalation products, and medical gases—the integrity of container closure systems, delivery devices, manufacturing components, and raw materials has never been more critical. The FDA’s recent guidances reflect this evolving reality and aim to tighten quality expectations across the entire product lifecycle. Three key guidances are: Current Good Manufacturing Practice for

Sharan Murugan
Nov 29, 20253 min read

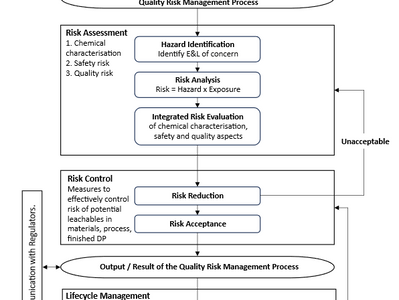
ICH Q3E – Impurities: Extractables and Leachables for Pharmaceuticals and Biologics
On 1 August 2025, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has...

Sharan Murugan
Aug 13, 20252 min read
