Search


USFDA Med Dev Guidance: Animal Studies for Dental Bone Grafting Material Devices in 510(k) Submissions
On 22 August 2025 , the U.S. Food and Drug Administration (FDA) released a comprehensive guidance document titled “ Animal Studies for...

Sharan Murugan
Aug 23, 20252 min read


UK MHRA Guidance: Applying for Clinical Trial Authorisation (CTA)
On 22 August 2025 , the Medicines and Healthcare products Regulatory Agency (MHRA) published an updated version of its guidance “...

Sharan Murugan
Aug 23, 20252 min read


USFDA Guidance: Predetermined Change Control Plans (PCCPs) for AI/ML Medical Devices
Artificial Intelligence and Machine Learning (AI/ML) are increasingly used in medical devices—from diagnostic imaging software to digital...

Sharan Murugan
Aug 19, 20252 min read


Malaysia–China Medical Device Regulatory Reliance Programme: Pilot Phase I (30 July – 30 September 2025)
The Medical Device Authority (MDA) of Malaysia has officially launched the Malaysia–China Medical Device Regulatory Reliance Programme ,...

Sharan Murugan
Aug 13, 20252 min read


European Commission Insights: Study on the Deployment of Artificial Intelligence in Healthcare – 2025
On 8 August 2025, the European Commission released an in-depth study on the " Deployment of artificial intelligence (AI) in healthcare...

Sharan Murugan
Aug 10, 20252 min read

UK MHRA Med Dev Guidance: Clinical Investigations for Medical Devices - What Sponsors Need to Know in 2025
Medical devices, whether diagnostic, therapeutic, or assistive, undergo rigorous scrutiny before entering the UK market. One of the most...

Sharan Murugan
Aug 6, 20253 min read

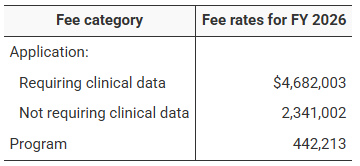
USFDA FDA User Fee Rates for FY 2026: A Comparative Overview for Prescription Drugs, Medical Devices, Generics, and Outsourcing Facilities
The U.S. Food and Drug Administration (FDA) has announced On 30 July 2025 , the official user fee rates across its major healthcare...

Sharan Murugan
Aug 2, 20252 min read


UK MHRA Guidance: Adverse Event Reporting in Digital Mental Health Technologies (DMHTs)
The proliferation of Digital Mental Health Technologies (DMHTs) —ranging from self-help apps and online therapy services to artificial...

Sharan Murugan
Aug 2, 20252 min read


USFDA Med Dev Guidance: Medical Device User Fee Small Business Qualification and Determination
In July 2025, the USFDA published a revised guidance titled " Medical Device User Fee Small Business Qualification and Determination "...

Sharan Murugan
Jul 31, 20252 min read


UK MHRA Guidance: Regulation of Medical Devices in Northern Ireland: Step-by-Step Guide (2025)
On 24 July 2025 , the UK Government published the latest update to its official guidance: " Regulation of medical devices in Northern...

Sharan Murugan
Jul 27, 20252 min read


USFDA's: FDA’s Commissioner’s National Priority Voucher (CNPV) Pilot Program to Accelerate Life-Changing Medicines
The U.S. Food and Drug Administration (FDA) has launched the " Commissioner’s National Priority Voucher (CNPV) Pilot Program " a...

Sharan Murugan
Jul 23, 20252 min read


Swissmedic Export Certificates: A Practical Guide for Medical Device Manufacturers
The efficient international supply of medical devices depends not only on innovation but on robust regulatory processes that certify...

Sharan Murugan
Jul 15, 20253 min read


UK Med Dev Guidance: How to Register Medical Devices with the MHRA and Digital Mental Health Technology Regulation
The regulatory landscape for medical devices and digital health technologies in the UK is evolving rapidly, reflecting the growing...

Sharan Murugan
Jul 5, 20253 min read


USFDA Guidance: UDI Requirements for Combination Products and Cybersecurity for Medical Devices
In June 2025, the FDA released two impactful draft guidances that significantly affect medical device and combination product...

Sharan Murugan
Jun 29, 20253 min read


MDCG Med Dev Guidance: Interplay between MDR & IVDR and the Artificial Intelligence Act
On June 19, 2025, the Medical Device Coordination Group (MDCG), in collaboration with the Artificial Intelligence Board (AIB) of the...

Sharan Murugan
Jun 28, 20253 min read


UK MHRA News: "AI Airlock" Pioneering Safe Healthcare Innovation in the UK
The UK’s Medicines & Healthcare products Regulatory Agency (MHRA) has launched Phase 2 of its AI Airlock programme , supported by a...

Sharan Murugan
Jun 23, 20252 min read


UK MHRA Guidances: Strengthening Device Oversight: Periodic Safety Update Reports (PSURs) for Medical Devices
In the ever-evolving landscape of medical device regulation, the Periodic Safety Update Report (PSUR) plays a pivotal role in ensuring...

Sharan Murugan
Jun 18, 20253 min read


UK MHRA Guidances: Ensuring Medical Device Safety in Great Britain: Field Safety Notices, Post-Market Surveillance, and Custom-Made Devices
In recent years, the MHRA has issued several key guidance documents strengthening the lifecycle safety and regulatory oversight of...

Sharan Murugan
Jun 18, 20253 min read


UK MHRA Guidance: Regulation of Medical Devices in Northern Ireland: What You Need to Know in 2025
The post-Brexit regulatory landscape for medical devices in the UK has brought significant changes, particularly in Northern Ireland,...

Sharan Murugan
Jun 13, 20252 min read


USFDA Draft Med.Dev Q&A: Transfer of a Premarket Notification (510(k)) Clearance – Questions and Answers
In June 2025, the U.S. Food and Drug Administration (FDA) released a new draft guidance titled “ Transfer of a Premarket Notification...

Sharan Murugan
Jun 7, 20252 min read
