USFDA FDA User Fee Rates for FY 2026: A Comparative Overview for Prescription Drugs, Medical Devices, Generics, and Outsourcing Facilities
- Sharan Murugan

- Aug 2, 2025
- 2 min read
The U.S. Food and Drug Administration (FDA) has announced On 30 July 2025, the official user fee rates across its major healthcare product categories for Fiscal Year 2026 (effective October 1, 2025–September 30, 2026). These fees, which manufacturers, sponsors, and facilities must pay for key regulatory submissions and annual operations, are central to supporting timely reviews, oversight, and safety monitoring in the US healthcare system.
PDUFA, first enacted in 1992 and currently in its seventh reauthorisation (PDUFA VII, FY 2023–2027), funds FDA’s review of new drug applications (NDAs) and biologics license applications (BLAs).
Prescription Drug User Fee Amendments (PDUFA) cover new and on-market branded drugs:
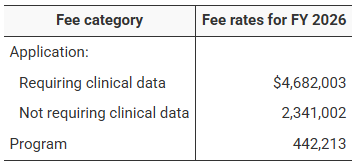
Application Fees: For new drug applications (NDAs/BLAs)
Requiring clinical data: $4,682,003
Not requiring clinical data: $2,341,002
Annual Program Fee: $442,213 per approved product

MDUFA, first passed in 2002, funds FDA’s review of medical device submissions (e.g., PMAs and 510(k)s).
Medical Device User Fee Amendments (MDUFA) cover devices including diagnostics, implants, and digital health products:
Annual Establishment Registration Fee: $11,423
Application Fees: Vary depending on submission type, for example:
510(k): $26,067 (standard); $6,517 (small business)
PMA, BLA: $579,272 (standard); $144,818 (small business)

GDUFA, launched in 2012 and now in its third reauthorisation, supports review of Abbreviated New Drug Applications (ANDAs).
Generic Drug User Fee Amendments (GDUFA) fund review and surveillance of generic medicines:
ANDA (Abbreviated New Drug Application) Fee: $358,247
DMF (Drug Master File) Fee: $102,584
Facility Fees:
Domestic API: $43,549
Foreign API: $58,077
Domestic FDF: $203,752
Foreign FDF: $218,311
Generic Drug Applicant Program Fees: Scale by company size; e.g., large firm: $1,534,701
4️⃣ Outsourcing Facility Fees (DQSA Section 503B)

Under the Drug Quality and Security Act (DQSA) of 2013, outsourcing facilities compound sterile drugs and register annually with FDA.
Compounding outsourcing facilities pay annual and reinspection fees:
Annual Establishment Fee:
Non-small business: $21,534
Small business: $6,488
Reinspection Fee: $19,465
✅ Why these updates matter
FDA user fees:
Fund faster, science-based product reviews
Support new IT systems, guidance, and patient engagement
Help industry plan annual budgets and timelines
Each program is tailored: PDUFA focuses on new innovator drugs; GDUFA targets generics; MDUFA covers medical devices; DQSA fees maintain compounding oversight.
For deeper details, tailored fee tables, or explanation of calculations, please click the below links above to consult the full Federal Register documents



Comments