Search

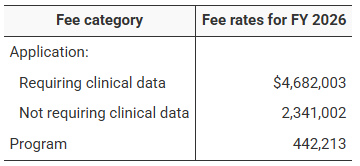
USFDA FDA User Fee Rates for FY 2026: A Comparative Overview for Prescription Drugs, Medical Devices, Generics, and Outsourcing Facilities
The U.S. Food and Drug Administration (FDA) has announced On 30 July 2025 , the official user fee rates across its major healthcare...

Sharan Murugan
Aug 2, 20252 min read


USFDA Guidance: Post-Warning Letter Meetings Under GDUFA III: A Regulatory Pathway Toward Compliance
In the evolving regulatory landscape of generic drug manufacturing, compliance and transparency are more crucial than ever. The FDA’s...

Sharan Murugan
Jun 18, 20252 min read


USFDA Guidance: Formal Meetings Between the FDA and Sponsors or Applicants -PDUFA Products
The FDA Center for Drug Evaluation and Research, along with the FDA Center for Biologics Evaluation and Research, issued a revised draft...

Sharan Murugan
Sep 21, 20232 min read


USFDA Guidance: Assessing User Fees Under the PDUFA of 2022
This Monday (01 May 2023) the United States Food & Drug Administration's Center for Drug Evaluation and Research and Center for Biologics...

Sharan Murugan
May 4, 20231 min read


FY2022 User Fee Table -USFDA
The User Fee programs help the Food and Drug Administration (FDA) to fulfill its mission of protecting public health and accelerating...

Sharan Murugan
Aug 13, 20212 min read
