Search

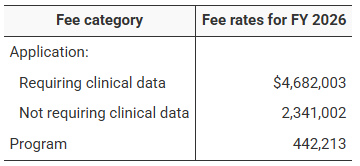
USFDA FDA User Fee Rates for FY 2026: A Comparative Overview for Prescription Drugs, Medical Devices, Generics, and Outsourcing Facilities
The U.S. Food and Drug Administration (FDA) has announced On 30 July 2025 , the official user fee rates across its major healthcare...

Sharan Murugan
Aug 2, 20252 min read


USFDA Guidance: Regulatory Considerations for Prescription Drug Use-Related Software
Yesterday (19 September 2023) the U.S. Food and Drug Administration (FDA) released two draft guidance "Regulatory Considerations for...

Sharan Murugan
Sep 20, 20232 min read


USFDA- Q&A Guide on Importation of Prescription Drugs from Canada
Yesterday, FDA issued a Small Entity Compliance Guide to help small businesses comply with the final rule on importation of prescription...

Sharan Murugan
May 26, 20221 min read
