Search


UK Med Dev Guidance: Clinical Investigations for Medical Devices
The Medicines and Healthcare products Regulatory Agency (MHRA) has published detailed guidance " Clinical Investigation s " on...

Sharan Murugan
Dec 4, 20242 min read


UK MHRA Press Release Trials Innovative AI Technologies in Regulatory Pilot Scheme
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) has launched (4th December 2024) a groundbreaking pilot scheme "...

Sharan Murugan
Dec 4, 20242 min read


MDCG Guidance: Implementation of the Master UDI-DI Solution for Contact Lenses
In November 2024, the Medical Device Coordination Group (MDCG) released " MDCG 2024-14 - Guidance on the implementation of the Master...

Sharan Murugan
Nov 30, 20242 min read


USFDA MD Guidance: Enhancing Safety and Efficiency in Orthopedic Devices and Sterilization Processes
The USFDA Center for Devices and Radiological Health has recently issued three key final guidance documents focusing on orthopedic...

Sharan Murugan
Nov 27, 20242 min read


Malaysia’s Medical Device Authority (MDA) Guidance: Control of Obsolete and Discontinued Medical Devices in Healthcare or Related Facilities
The Medical Device Authority (MDA) in Malaysia has released a comprehensive guidance " Control of Obsolete and Discontinued Medical...

Sharan Murugan
Nov 20, 20242 min read

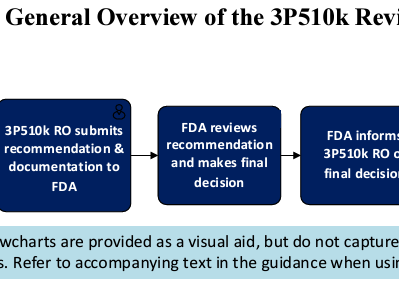
USFDA Guidance: 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review
The 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review are pivotal initiatives by the USFDA to...

Sharan Murugan
Nov 20, 20242 min read


Swissmedic Guidance on Product Information for Human Medicinal Products
Swissmedic, the Swiss Agency for Therapeutic Products, has introduced its updated guidance on " Product Information for Human Medicinal...

Sharan Murugan
Nov 16, 20242 min read


Swissmedic Guidance: Combined Studies: Clinical Investigations of Medical Devices, Medicinal Products, and Advanced Therapy Medicinal Products (ATMPs)
The Swissmedic guidance released an information sheet titled " Combined Studies: Clinical Investigations of Medical Devices, Medicinal...

Sharan Murugan
Nov 9, 20242 min read


SwissMedic Med Dev: Clinical Investigations of Medical Devices & Performance Studies of In Vitro Diagnostics (IVD)
The Swissmedic released (31 October, 2024) updated guidance on " Clinical Investigations with Medical Devices " and " Performance studies...

Sharan Murugan
Nov 3, 20242 min read


Australia's TGA Good Clinical Practice (GCP) Inspection Program
Recently last week (30 October, 2024) the TGA released an updated reference material about the " Good Clinical Practice (GCP) Inspection...

Sharan Murugan
Nov 3, 20242 min read


MDCG Guidance: MDR requirements for Legacy Devices
The Medical Device Coordination Group (MDCG) has released guidance " Application of MDR requirements to ‘legacy devices’ and to devices...

Sharan Murugan
Oct 27, 20242 min read


MDCG Med Dev: Guidance and Templates for Conformity Assessment Bodies, Notified Bodies, Designating Authorities, and Joint Assessment Teams
The Medical Device Coordination Group (MDCG) has released its updated guidance and templates for conformity assessment bodies, notified...

Sharan Murugan
Oct 14, 20242 min read


MDCG Med. Dev Guidance: Qualification of In Vitro Diagnostic Medical Devices (IVDs)
The Medical Device Coordination Group (MDCG) has released a detailed guidance document titled " Qualification of In Vitro Diagnostic...

Sharan Murugan
Oct 14, 20242 min read


Australia TGA: eCTD AU Module 1 and Regional Information v3.2
The Therapeutic Goods Administration (TGA) of Australia has released the latest version, v3.2, (on 13 September 2024) of the eCTD AU...

Sharan Murugan
Oct 2, 20242 min read


USFDA Medical Device: Guidance on Dental Products (Dental Impression Materials, Dental Ceramics, Dental Cements and Air Powered Dental Handpieces and Air Motors)
USFDA's Center for Devices and Radiological Health has released four final guidances about dental products, such as Dental Impression...

Sharan Murugan
Sep 28, 20242 min read


India CDSCO Guidance: Pharmacovigilance Document for Marketing Authorization Holders of Pharmaceutical Products
India's CDSCO Pharmacovigilance released an updated guidance " Pharmacovigilance Guidance Document for Marketing Authorization Holders of...

Sharan Murugan
Sep 25, 20242 min read


USFDA Med Dev Guidance: Biocompatibility Testing and Accreditation Scheme for Conformity Assessment (ASCA) Program
USFDAs Center for Devices and Radiological Health and Center for Biologics Evaluation and Research released multiple draft guidance...

Sharan Murugan
Sep 22, 20242 min read


USFDA Guidance: Appeal Options for Mammography Facilities
The USFDA (U.S. Food and Drug Administration) Center for Devices and Radiological Health provides detailed final guidance " Appeal...

Sharan Murugan
Sep 15, 20242 min read


EMA: Harnessing AI in Medicines Regulation: The Role of Large Language Models (LLMs)
The advancement of Artificial Intelligence (AI) is transforming many industries, and the pharmaceutical and regulatory sectors are no...

Sharan Murugan
Sep 15, 20242 min read


USFDA Guidance: Incorporating Voluntary Patient Preference Information Over the Total Product Life Cycle
USFDA's Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER) released a draft...

Sharan Murugan
Sep 6, 20242 min read
