Search


European Commission Insights: Study on the Deployment of Artificial Intelligence in Healthcare – 2025
On 8 August 2025, the European Commission released an in-depth study on the " Deployment of artificial intelligence (AI) in healthcare...

Sharan Murugan
Aug 10, 20252 min read

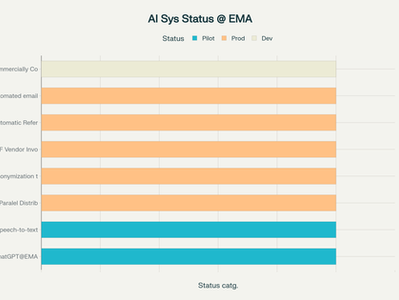
EMA’s AI Journey: The Rise of Artificial Intelligence in Medicines Regulation
In a world where artificial intelligence (AI) is moving rapidly from theory to practice, regulators are tasked with keeping pace—not only...

Sharan Murugan
Jul 19, 20253 min read


CIOMS Working Group Draft Report: Artificial Intelligence in Pharmacovigilance: Key Takeaways
The field of pharmacovigilance (PV) —the science of detecting, assessing, understanding, and preventing adverse effects or any other...

Sharan Murugan
May 13, 20253 min read


USFDA Guidance: Artificial Intelligence-Enabled Device Software Functions – Lifecycle Management and Marketing Submission Recommendations
The U.S. Food and Drug Administration (FDA) has issued a draft guidance document titled “ Artificial Intelligence-Enabled Device Software...

Sharan Murugan
Jan 7, 20252 min read


USFDA Draft Guidance: Considerations for the Use of Artificial Intelligence to Support Regulatory Decision
The U.S. Food and Drug Administration (FDA) released a draft guidance earlier today (07 January, 2025) outlining " Considerations for...

Sharan Murugan
Jan 7, 20252 min read


New EU Regulations on AI in Medical Devices: Key Insights and Implications
The European Union has published the finalized text of the Artificial Intelligence Act (AIA) which is a significant step forward in...

Sharan Murugan
Jul 15, 20242 min read


IMDRF Guidance: Good Machine Learning Practice for Medical Device Development: Guiding Principles
The International Medical Device Regulators Forum (IMDRF) has published a draft guidance (01 July, 2024) "Good machine learning practice...

Sharan Murugan
Jul 3, 20242 min read


USFDA's ISTAND Pilot Program: Accepts First Submission of AI-Based Digital Tech for Neuroscience
A new submission has been accepted into the FDA's Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program by...

Sharan Murugan
Jan 23, 20242 min read


WHO Guidance: Ethics and Governance of Artificial Intelligence for Health
The World Health Organization (WHO) released new guidelines (18 January 2023) on ethics and governance of large multi-modal models...

Sharan Murugan
Jan 20, 20241 min read


UK MHRA: Guidance on Software and Artificial Intelligence (AI) as a Medical Device
Earlier today (25 October 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated Guidance on "Software...

Sharan Murugan
Oct 25, 20231 min read


USFDA Med Dev: Marketing Submission Recommendations for Change Control Plan for AI/ML Enabled Device
Earlier today (30 March 2023) USFDA released draft guidance on "Marketing Submission Recommendations for a Predetermined Change Control...

Sharan Murugan
Mar 30, 20231 min read


Artificial Intelligence in Regulatory Affairs
Artificial Intelligence (AI) is a broad term that encompasses many different things and is a kind of intelligence that is created by a...

Sharan Murugan
Sep 21, 20223 min read
