Search


Health Canada: Guidance on Validation rules for Regulatory Transactions in the eCTD Format
Health Canada recently published an updated guidance on "Validation rules for regulatory transactions provided to Health Canada in the...

Sharan Murugan
Nov 30, 20231 min read


Swiss Medic: Biosimilar Authorisation Guidance
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance on (26 November 2023) the "Guidance document...

Sharan Murugan
Nov 26, 20232 min read


UK MHRA: Guidance on Change of Ownership: Marketing Authorisation Process
Last Friday (17 November 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Medicines...

Sharan Murugan
Nov 18, 20232 min read

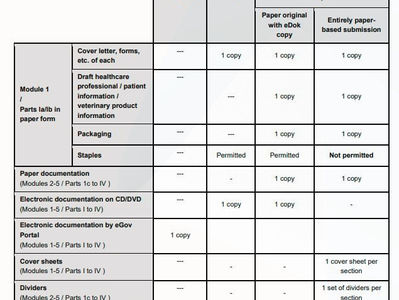
Switzerland's SwissMedic: Guidance on Formal Requirements
On 15 November 2023, Switzerland's Swissmedic released an updated guidance on "Formal Requirements" which serves as a resource for...

Sharan Murugan
Nov 15, 20232 min read


USFDA Guidance: Real-Time Oncology Review (RTOR)
The U.S. Food and Drug Administration (FDA) Oncology Center of Excellence (OCE) released guidance earlier this week (07 November 2023)...

Sharan Murugan
Nov 11, 20232 min read


MHRA Guidance: Common Issues Identified during Clinical Trial Applications
Yesterday (06 November 2023) the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Common...

Sharan Murugan
Nov 7, 20231 min read


USFDA Guidance: Supplements for Approved Premarket Approval or Humanitarian Device Exemption
Today (02 November, 2023) the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research Center for Devices and...

Sharan Murugan
Nov 2, 20231 min read


TGA Guidance: General Dossier Requirements
Recently on 27th October, 2023 Australia's Therapeutic Goods Administration (TGA) released an updated guidance on the "General Dossier...

Sharan Murugan
Oct 31, 20231 min read


USFDA: Remote Interactive Evaluations of Drug Manufacturing & Bioresearch Monitoring Facilities
Earlier today (25 October,2023), the USFDA issued a draft guidance, "Remote Interactive Evaluations of Drug Manufacturing and Bioresearch...

Sharan Murugan
Oct 25, 20231 min read


USFDA MD Guidance: Enforcement Policy for Non-Invasive Remote Monitoring Devices
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health released a final guidance "Enforcement Policy for...

Sharan Murugan
Oct 23, 20231 min read


USFDA Guidance: Benefit-Risk Assessment for New Drug and Biological Products
A final guidance "Benefit-Risk Assessment for New Drug and Biological Products" was published last week (17 October, 2023) by the US Food...

Sharan Murugan
Oct 23, 20232 min read


EMA Guidance: Procedural Advice for Orphan Medicinal Product Designation
Yesterday (20 October 2023) the European Medicines Agency released an updated guidance on "Procedural Advice for Orphan Medicinal Product...

Sharan Murugan
Oct 21, 20232 min read


UK MHRA Guidance: Apply for a Licence to Market a Medicine in the UK
Last Thursday (19 October 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Apply for a...

Sharan Murugan
Oct 21, 20231 min read


USFDA Guidance: Policy for Testing of Alcohol & Developing Drugs for DFI Treatment
Earlier today (17 October 2023) the United States Food & Drug Administration's Center for Drug Evaluation and Research and Center for...

Sharan Murugan
Oct 17, 20232 min read


EMA Guidance: Post-Authorisation Procedural Advice for Users of the Centralised Procedure
Yesterday (13 October 2023) the European Medicines Agency released an updated guidance on "European Medicines Agency post-authorisation...

Sharan Murugan
Oct 14, 20231 min read


UK MHRA: Clinical Trials for Medicines: Apply for Authorisation in the UK
Recently (12 October 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Clinical Trials...

Sharan Murugan
Oct 14, 20232 min read


EMA Guidance: Qualification of Novel Methodologies for Drug Development, Guidance to Applicants
Recently the European Medicines Agency released an updated guidance on "Qualification of Novel Methodologies for Drug Development:...

Sharan Murugan
Oct 10, 20232 min read


USFDA Guidance: Dose Banding - Labeling for Dosing Based on Weight or Body Surface Area
Earlier today (2 October 2023), The U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research, Center for Biologics...

Sharan Murugan
Oct 2, 20232 min read


EC Q&A Guidance: Questions & Answers: Clinical Trials Regulation
Recently this Friday (29-Sepetember, 2023), the European Commission's Clinical Trials Coordination and Advisory Group released an updated...

Sharan Murugan
Oct 1, 20232 min read


MHRA Guidance: The Northern Ireland MHRA Authorised Route (NIMAR)
On 29 September 2023 UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "The Northern Ireland...

Sharan Murugan
Oct 1, 20231 min read
