Search


UK MHRA Guidance: UK Clinical Trials Regulatory Guidance – A Complete Overview (2026)
In the United Kingdom, the regulatory landscape operates under a well-defined set of guidance documents established and maintained by the UK Government and the Medicines and Healthcare products Regulatory Agency (MHRA). These documents help sponsors, regulatory affairs teams, and clinical operations professionals comply with UK law while ensuring patient safety and data integrity. In 2026, several key guidances outline the authorisation process, trial notifications, reporting

Sharan Murugan
Jan 313 min read


UK MHRA Guidance: Clinical Trials for Medicines: Apply for Authorisation in the UK & Early Access to Medicines Scheme (EAMS)
The UK regulatory environment now offers two important pathways for bringing innovative medicinal therapies to patients: The Early Access to Medicines Scheme (EAMS) , which permits earlier availability of new medicines under conditions of unmet medical need; and The MHRA’s guidance “ Clinical trials for medicines: apply for authorisation in the UK ” , which provides the regulatory framework for initiating clinical trials of investigational medicinal products. These 2 guidance

Sharan Murugan
Nov 15, 20252 min read


USFDA Guidance: Integrating Patient-Focused Development, Expanded Access, and Clinical Data Specifications in the Evolving FDA Framework
The U.S. Food and Drug Administration (FDA) continues to refine its regulatory guidance structure to ensure that patient experience, ethical access pathways, and robust data science converge effectively in modern drug development. Three cornerstone guidance documents released through 2024–2025 exemplify this integration — focusing on patient-focused drug development, expanded access to investigational drugs, and technical specifications for clinical trial data submissions. 1.

Sharan Murugan
Oct 26, 20253 min read


MHRA Updates Comprehensive Guidance Framework for Clinical Trials in the UK (October 2025)
The UK’s Medicines and Healthcare products Regulatory Agency ( MHRA ) has issued a coordinated update to its suite of clinical trial...

Sharan Murugan
Oct 5, 20253 min read


Swissmedic Guidance: Clinical Trials with Medicinal Products, Submission Process and FAQs
Clinical trials are the cornerstone of developing safe and effective medicines. In Switzerland, the regulatory authority...

Sharan Murugan
Aug 28, 20252 min read


Swissmedic Guidance: Submission Process for Clinical Trials with Medicinal Products
On 18 August 2025, Swissmedic published Version 2.3 of its guidance on the " Submission Process for Clinical Trials with Medicinal...

Sharan Murugan
Aug 23, 20252 min read


India CDSCO Guidance: Subject Expert Committee (SEC), What Pharma and Clinical Trial Teams Need to Know
The Central Drugs Standard Control Organization (CDSCO) under India’s Ministry of Health & Family Welfare has released a detailed...

Sharan Murugan
Jul 23, 20252 min read

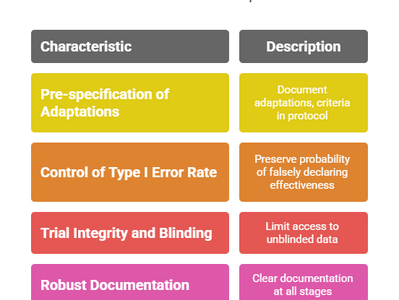
ICH E20 Draft Guideline: Understanding Adaptive Clinical Trials in Focus (2025)
The International Council for Harmonisation (ICH) has taken a major step toward modernizing clinical trial designs with the release of the...

Sharan Murugan
Jun 28, 20253 min read


UK MHRA Guidances: 9 important Guidances on Clinical Trials Safety, Approvals, Labelling, and More
Clinical trials are the cornerstone of modern medicine, ensuring that new treatments are safe, effective, and suitable for public use....

Sharan Murugan
Jun 28, 20253 min read


Swissmedic’s Clinical Trial Guidance Suite: Everything Sponsors Need to Know
On June 2, 2025 , Swissmedic published a harmonised suite of guidance documents to standardise, streamline, and digitalise the clinical...

Sharan Murugan
Jun 7, 20252 min read


MHRA Guidance: Good Clinical Practice (GCP) for Clinical Trials
The Medicines and Healthcare Products Regulatory Agency (MHRA) has released updated guidance on " Good Clinical Practice (GCP) for...

Sharan Murugan
Mar 15, 20253 min read


India CDSCO: Adding Trial Sites and Changing Principal Investigators
Clinical trials are a cornerstone of medical advancement, requiring meticulous planning and adherence to regulatory guidelines. In India,...

Sharan Murugan
Mar 3, 20253 min read


UK MHRA Guidance: Managing Clinical Trial Authorisations & Safety Reporting (Updated 2025)
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has updated its guidance on " Clinical trials for medicines: manage...

Sharan Murugan
Feb 9, 20252 min read


USFDA Guidance: Developing Drugs for Optical Imaging – A Comprehensive Insight
On 07 January 2025, the U.S. Food and Drug Administration (FDA) released the draft guidance titled " Developing Drugs for Optical Imaging...

Sharan Murugan
Jan 11, 20252 min read


USFDA Guidance: Technical Specifications for Submitting Clinical Trial Data Sets for Treatment of Noncirrhotic Nonalcoholic Steatohepatitis (NASH)
The U.S. Food and Drug Administration (FDA) has released a guidance " Technical Specifications for Submitting Clinical Trial Data Sets...

Sharan Murugan
Dec 15, 20242 min read


USFDA Guidance: Core Patient-Reported Outcomes in Cancer Clinical Trials & Considerations for Long-Term Clinical Neurodevelopmental Safety Studies in Neonatal Product Development
The U.S. Food and Drug Administration (USFDA) yesterday (17 October, 2024) has issued two comprehensive Q&A guidance titled " Core...

Sharan Murugan
Oct 17, 20242 min read

SFDA Guidance: Conducting Clinical Trials with Decentralized Elements & Integrating Randomized Controlled Trials
Guidance: Conducting Clinical Trials With Decentralized Elements The U.S. Food and Drug Administration (FDA) Center for Drug Evaluation...

Sharan Murugan
Sep 22, 20242 min read


UK MHRA Guidance: Clinical Trials for Medicines – Applying for Authorisation in the UK
The UK Medicines and Healthcare products Regulatory Agency (MHRA) updated their comprehensive guidance " Clinical Trials for Medicines –...

Sharan Murugan
Aug 27, 20242 min read


UK MHRA: Guidance on Good Clinical Practice for Clinical Trials
On 22nd July 2024 the UK Medicines and Healthcare Products Regulatory Agency (MHRA) issued comprehensive guidance on " Good Clinical...

Sharan Murugan
Jul 23, 20242 min read


USFDA Guidance: Cancer Clinical Trial Eligibility Criterias
The U.S. Food and Drug Administration (FDA) Oncology Center of Excellence, Center for Drug Evaluation and Research, and Center for...

Sharan Murugan
Apr 27, 20242 min read
