Search

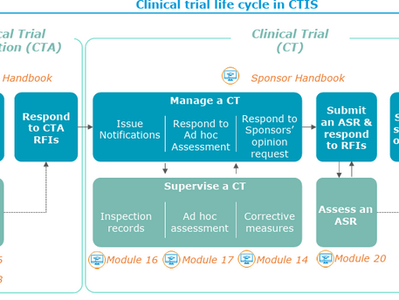
EMA’s CTIS Framework: Decoding the EMA Clinical Trial Information System-A Practical Guide for Sponsors
In an era of data-driven drug development, the European Medicines Agency (EMA) has launched a transformative regulatory framework : the...

Sharan Murugan
Jul 9, 20252 min read


EMA Interim Guidance: How to Approach the "PPD and CCI" while using CTIS
Last Week (03 May 2023) the European Medicines Agency released an Interim guidance document on "How to Approach the Protection of...

Sharan Murugan
May 7, 20232 min read


Ireland's HPRA: Guide to Clinical Trial Applications
Recently in January, the Health Products Regulatory Authority (HPRA) of Ireland updated its guidance on "HPRA Guide to Clinical Trial...

Sharan Murugan
Feb 2, 20231 min read


Clinical Trials Information System (CTIS) to go-live in 6 Months – EMA
The European Commission has confirmed that the entry into application of the Clinical Trials Regulation and hence the go-live date for...

Sharan Murugan
Aug 3, 20211 min read


Clinical Trials Information System (CTIS) Free Webinar – EMA
Clinical Trials Information System (CTIS) will act as the single entry point for clinical trials authorisation and supervision in the...

Sharan Murugan
Jul 25, 20211 min read
