Search


USFDA Guidance: Use of Artificial Intelligence To Support Regulatory Decision & Guiding Principles of Good AI Practice in Drug Development
Artificial Intelligence (AI) is no longer a future concept in pharmaceutical research—it is already influencing how drugs and biological products are discovered, developed, manufactured, and monitored throughout their lifecycle. However, when AI outputs are used to support regulatory decisions , questions of trust, transparency, and accountability become critical. Recognising this, global regulators have begun defining expectations for the responsible use of AI in drug devel

Sharan Murugan
Jan 313 min read


EMA Overview: European Shortages Monitoring Platform (ESMP): How the EU Monitors, Prevents, and Manages Medicine Shortages
Medicine shortages can have a direct and serious impact on patient care, healthcare systems, and public health across Europe. To strengthen coordination, improve visibility, and enable early action, the European Medicines Agency (EMA) has established the European Shortages Monitoring Platform (ESMP) . The ESMP is a central digital platform designed to support the prevention, identification, monitoring, and management of shortages of medicinal products across the EU and EEA.

Sharan Murugan
Dec 20, 20253 min read


EMA Draft Guideline on Quality of Radiopharmaceuticals
Radiopharmaceuticals present unique quality challenges due to their radioactive nature, short shelf lives, and frequent need for on-site or near-patient preparation. To reflect scientific and technological advances since the original 2007 guideline, the European Medicines Agency has released the draft Guideline on quality of radiopharmaceuticals – Revision 2 for public consultation. Adopted by CHMP in December 2025, this revision provides updated expectations for quality doc

Sharan Murugan
Dec 13, 20252 min read


EMA Guideline on the Development, Manufacture of Synthetic Peptides and Good Pharmacogenomic Practice
Modern medicines development increasingly sits at the intersection of advanced chemistry and genomic science. On one hand, synthetic peptides represent a fast-growing therapeutic class that bridges small molecules and biologics. On the other, pharmacogenomics is reshaping how medicines are developed, evaluated, and ultimately used in patients. Recognising these parallel advances, the European Medicines Agency (EMA) has recently published two key scientific documents: the Guid

Sharan Murugan
Dec 13, 20253 min read


EMA Guideline on Stability Testing for Applications for Variations to a Marketing Authorisation
Post-authorisation changes are an inevitable part of medicinal product lifecycle management, and stability data play a critical role in demonstrating that such changes do not compromise product quality, safety, or efficacy. To harmonise expectations across the EU, the European Medicines Agency has issued Revision 3 of the Guideline on stability testing for applications for variations to a marketing authorisation . Adopted in December 2025 and effective from January 2026, this

Sharan Murugan
Dec 13, 20252 min read


EMA Q&A Guidance: Updated Classification of (Post-Authorisation) Changes
Regulatory teams responsible for lifecycle management know that post-authorisation changes can quickly become complex, especially as products mature, manufacturing processes evolve, and emerging scientific data require frequent updates to quality and clinical documentation. To support consistent interpretation of EU Variation Regulation (EC) No 1234/2008, the European Medicines Agency (EMA) maintains an extensive Q&A document titled " Classification of Changes " —a practical

Sharan Murugan
Dec 7, 20254 min read


EMA Guidance: ETF Scientific Advice that facilitates Clinical Trial Authorisations (SA-CTA) and 2025–2027 IRIS Roadmap
The European Medicines Agency (EMA) recently published new guidance titled “ Guidance for applicants: the ETF Scientific Advice that facilitates Clinical Trial Authorisations (SA-CTA ) ”, outlining how sponsors and applicants can benefit from harmonised scientific advice to accelerate clinical trial authorisation across the EU. 1. What the SA-CTA Scientific Advice Is The guidance explains that the SA-CTA scientific advice is a specialised type of EMA advice developed under th

Sharan Murugan
Nov 23, 20254 min read


EMA’s New Draft Guideline: Non-Inferiority and Equivalence Comparisons in Clinical Trials
The European Medicines Agency (EMA) has released a major draft guideline " Non-Inferiority and Equivalence Comparisons in Clinical Trials " that updates how non-inferiority and equivalence comparisons should be designed, justified, and analysed in confirmatory clinical trials. This draft replaces two earlier documents: Guideline on the choice of the non-inferiority margin (2005), and Points to consider on switching between superiority and non-inferiority (2000). The new gu

Sharan Murugan
Nov 17, 20252 min read


EMA Guidance: Regulating Innovation in Phage Therapy and Device–Drug Combinations
In October 2025, the European Medicines Agency (EMA) released two landmark drafts for public consultation. These documents collectively signal the Agency’s evolving regulatory stance toward precision biologics such as bacteriophage therapy and innovative device–drug integration approaches to simplify clinical bridging for biologics delivered subcutaneously. 1. Quality Guidance for Phage Therapy Medicinal Products (PTMPs) The EMA draft guideline on phage therapy quality (Oct

Sharan Murugan
Oct 26, 20252 min read


EMA Guidance: European Commission’s (EC) New Variations Guidelines
On 22 September 2025 , the European Commission published new variations guidelines in the Official Journal of the European Union. These...

Sharan Murugan
Sep 22, 20252 min read


EMA Publishes First EU eCTD v4.0 Validation Criteria and Updated Controlled Vocabularies
In 1st & 8th August 2025, the European Medicines Agency (EMA) announced two major milestones in the transition to electronic Common...

Sharan Murugan
Aug 10, 20252 min read


EMA Procedural Advice: Paediatric Applications – A Comprehensive Guide for Applicants
On 8 August 2025 , the European Medicines Agency (EMA) published the latest revision (Rev. 14) of its "Procedural Advice on Paediatric...

Sharan Murugan
Aug 10, 20252 min read

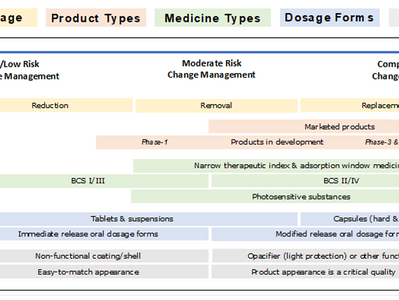
EMA’s Feedback: On Replacing Titanium Dioxide (TiO₂) in Medicinal Products: Critical Challenges, Limited Alternatives (Human & Veterneary)
The European Medicines Agency (EMA) submitted its updated report to the European Commission (EC) evaluating the feasibility of...

Sharan Murugan
Aug 6, 20253 min read


EMA Guidance: Procedural Advice on Paediatric Applications
On 25 July 2025 , the European Medicines Agency (EMA) published the latest guidance " Procedural Advice on Paediatric Applications " ....

Sharan Murugan
Jul 31, 20252 min read

EMA Guidance: Requirements for Demonstrating Therapeutic equivalence between Orally Inhaled Products (OIP) and Chronic Obstructive Pulmonary Disease (COPD)
On 14 July 2025 , the European Medicines Agency (EMA) published the final revised guideline titled " Guideline on the requirements for...

Sharan Murugan
Jul 23, 20252 min read

European Commission: Guidelines on the Scope of the Obligations for General-Purpose AI Models established by AI Act
On 18 July 2025, the European Commission published detailed Guidelines on the scope of the obligations for providers of general-purpose...

Sharan Murugan
Jul 19, 20253 min read

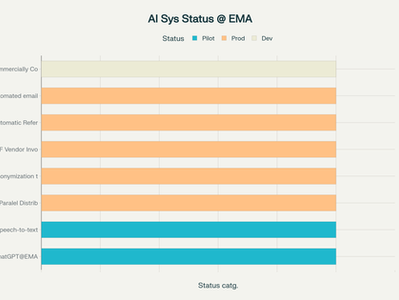
EMA’s AI Journey: The Rise of Artificial Intelligence in Medicines Regulation
In a world where artificial intelligence (AI) is moving rapidly from theory to practice, regulators are tasked with keeping pace—not only...

Sharan Murugan
Jul 19, 20253 min read


EMA Guidance: Procedural Advice for Orphan Medicinal Product Designation
The European Medicines Agency (EMA) provides structured guidance for sponsors aiming to secure orphan medicinal product designation...

Sharan Murugan
Jul 19, 20252 min read


EMA IRIS Guide: How to Create, Submit and Manage IRIS applications, for Industry and Individual applicants
In the ever-evolving regulatory landscape of EU pharmaceuticals, digital solutions are a linchpin for timely and transparent scientific...

Sharan Murugan
Jul 15, 20252 min read

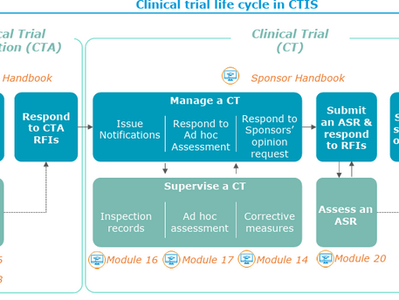
EMA’s CTIS Framework: Decoding the EMA Clinical Trial Information System-A Practical Guide for Sponsors
In an era of data-driven drug development, the European Medicines Agency (EMA) has launched a transformative regulatory framework : the...

Sharan Murugan
Jul 9, 20252 min read
