Search

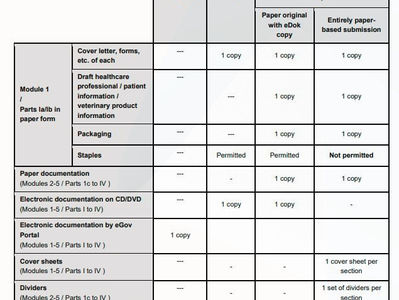
Switzerland's SwissMedic: Guidance on Formal Requirements
On 15 November 2023, Switzerland's Swissmedic released an updated guidance on "Formal Requirements" which serves as a resource for...

Sharan Murugan
Nov 15, 20232 min read


MHRA Guidance: Common Issues Identified during Clinical Trial Applications
Yesterday (06 November 2023) the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Common...

Sharan Murugan
Nov 7, 20231 min read


USFDA MD Guidance: Enforcement Policy for Clinical Electronic Thermometers
Last Friday (03 November 2023) the U.S. Food and Drug Administration (FDA) released two draft guidance "Enforcement Policy for Clinical...

Sharan Murugan
Nov 5, 20231 min read


USFDA Guidance: Supplements for Approved Premarket Approval or Humanitarian Device Exemption
Today (02 November, 2023) the U.S. Food and Drug Administration (FDA) Center for Biologics Evaluation and Research Center for Devices and...

Sharan Murugan
Nov 2, 20231 min read


TGA Guidance: General Dossier Requirements
Recently on 27th October, 2023 Australia's Therapeutic Goods Administration (TGA) released an updated guidance on the "General Dossier...

Sharan Murugan
Oct 31, 20231 min read


UK MHRA: Guidance on Software and Artificial Intelligence (AI) as a Medical Device
Earlier today (25 October 2023) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated Guidance on "Software...

Sharan Murugan
Oct 25, 20231 min read


USFDA MD Guidance: Enforcement Policy for Non-Invasive Remote Monitoring Devices
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health released a final guidance "Enforcement Policy for...

Sharan Murugan
Oct 23, 20231 min read


USFDA MD Guidance: Submission of Premarket Notifications for Magnetic Resonance Diagnostic Devices
Recently (10th October 2023) the U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health released multiple...

Sharan Murugan
Oct 14, 20231 min read


USFDA MD Guidance: AST System Devices, Technical Considerations & Electronic Submission Template
Recently (29th September 2023) the U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health released multiple...

Sharan Murugan
Oct 2, 20232 min read


USFDA MD Guidance: Cybersecurity-Quality System Considerations & Content of Premarket Submissions
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health released a final guidance "Cybersecurity in...

Sharan Murugan
Oct 2, 20231 min read


EC Q&A Guidance: Questions & Answers: Clinical Trials Regulation
Recently this Friday (29-Sepetember, 2023), the European Commission's Clinical Trials Coordination and Advisory Group released an updated...

Sharan Murugan
Oct 1, 20232 min read


MHRA MD Guidance: Innovative Devices Access Pathway (IDAP) & How to Notify Clinical Investigation
Recently the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "The Innovative Devices Access...

Sharan Murugan
Sep 25, 20232 min read


USFDA Guidance: Conduct of Clinical Trials of Medical Products During Major Disruptions
Today (21 September 2023) CDER, along with the FDA Center for Biologics Evaluation and Research, Center for Devices and Radiological...

Sharan Murugan
Sep 21, 20231 min read


USFDA Guidance: Regulatory Considerations for Prescription Drug Use-Related Software
Yesterday (19 September 2023) the U.S. Food and Drug Administration (FDA) released two draft guidance "Regulatory Considerations for...

Sharan Murugan
Sep 20, 20232 min read


USFDA M.D Guidance: Med Dev Associated with Weight Loss & Voluntary Improvement Program
Recently (14th September, 2023) the U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health released multiple...

Sharan Murugan
Sep 19, 20232 min read
![USFDA Guidance: Premarket Notification [510(k)] Submissions](https://static.wixstatic.com/media/nsplsh_453363554d4e4263424363~mv2.jpg/v1/fill/w_333,h_250,fp_0.50_0.50,q_30,blur_30,enc_avif,quality_auto/nsplsh_453363554d4e4263424363~mv2.webp)
![USFDA Guidance: Premarket Notification [510(k)] Submissions](https://static.wixstatic.com/media/nsplsh_453363554d4e4263424363~mv2.jpg/v1/fill/w_399,h_300,fp_0.50_0.50,q_90,enc_avif,quality_auto/nsplsh_453363554d4e4263424363~mv2.webp)
USFDA Guidance: Premarket Notification [510(k)] Submissions
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health and Center for Biologics Evaluation and Research...

Sharan Murugan
Sep 10, 20232 min read


Australia's TGA: Reclassification of Medical Devices & Variations to Prescription Medicines
Yesterday (21-August-2023) Australia's Therapeutic Goods Administration (TGA) released multiple guidances on the "Reclassification of...

Sharan Murugan
Aug 22, 20232 min read


USFDA Guidance: Informed Consent Guidance for IRBs, Clinical Investigators, & Sponsors
The U.S. Food and Drug Administration (FDA) released its final guidance this Tuesday (15 August 2023), "Informed Consent: Guidelines for...

Sharan Murugan
Aug 17, 20231 min read


USFDA Med Dev Guidance: On Opioid Use Disorder & Hydrogen Peroxide-Based Contact Lens Care Products
Clinical Considerations for Studies of Devices Intended to Treat Opioid Use Disorder Last week (26 June, 2023) the United States Food &...

Sharan Murugan
Jul 30, 20232 min read


UK MHRA: Guidance on Renewing Marketing Authorisations & Implementation of Med Dev. Future Regime
Guidance on Renewing Marketing Authorisations Recently (28, July 2023) the UK's Medicines and Healthcare Products Regulatory Agency...

Sharan Murugan
Jul 30, 20231 min read
