Search


UK MHRA Guidance: Regulation of Medical Devices in Northern Ireland: Step-by-Step Guide (2025)
On 24 July 2025 , the UK Government published the latest update to its official guidance: " Regulation of medical devices in Northern...

Sharan Murugan
Jul 27, 20252 min read

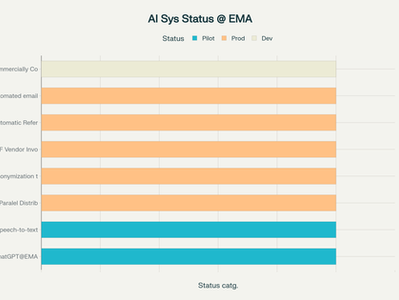
EMA’s AI Journey: The Rise of Artificial Intelligence in Medicines Regulation
In a world where artificial intelligence (AI) is moving rapidly from theory to practice, regulators are tasked with keeping pace—not only...

Sharan Murugan
Jul 19, 20253 min read


UK MHRA Guidance: Regulation of Medical Devices in Northern Ireland: What You Need to Know in 2025
The post-Brexit regulatory landscape for medical devices in the UK has brought significant changes, particularly in Northern Ireland,...

Sharan Murugan
Jun 13, 20252 min read


UK Medical Device Regulations: Registration and Export Guidelines
Earlier today the Medicines and Healthcare products Regulatory Agency (MHRA) released updated guidance " Register medical devices to...

Sharan Murugan
Mar 13, 20253 min read


Navigating UK MHRA Guidance: Registration and Regulation of Medical Devices in the UK
The Medicines and Healthcare Products Regulatory Agency (MHRA) oversees the regulation and registration of medical devices within the...

Sharan Murugan
Feb 18, 20252 min read
