Search


Swissmedic Guidance: Requesting Product Certificates (CPP)
Swissmedic, the Swiss Agency for Therapeutic Products, has issued a detailed guidance document "Requesting Certificates of Pharmaceutical...

Sharan Murugan
Jun 23, 20242 min read


Swissmedic Guidance: Renewal and Discontinuation of Authorization or Change of Status (Main Authorization/Export License)
Swissmedic, the Swiss Agency for Therapeutic Products, has published comprehensive guidance "Renewal and Discontinuation of Authorization...

Sharan Murugan
Jun 15, 20242 min read


Swiss Medic: Guidances on Varaiations, Temporary Authorisation, Meetings for Applicants and Time Limits for Authorisation
Swissmedic, the Swiss Agency for Therapeutic Products, is responsible for ensuring that only high-quality, safe, and effective medical...

Sharan Murugan
Jun 9, 20242 min read


Switzerland’s Swissmedic: Guidance on Temporary Authorization to Use an Unauthorised Medicinal Product
Swissmedic, the Swiss Agency for Therapeutic Products, has provided guidelines on (15 May 2024) "Temporary Authorization to Use an...

Sharan Murugan
May 18, 20242 min read


Switzerland's SwissMedic: Guidance for requesting Product Certificates (CPP)
On 3rd May 2024 Switzerland's Swissmedic released an updated guidance document "For requesting Product Certificates (CPP)" that guides in...

Sharan Murugan
May 6, 20242 min read


Swissmedic Guidance: Import of a Human Medicinal Product (Parallel Import)
Today (01 May 2024) Switzerland's Swissmedic released updated guidance on "Import of a Human Medicinal Product according to Art. 14 para....

Sharan Murugan
May 1, 20242 min read


Switzerland's SwissMedic: Guidance on Export Certificates
On 3rd April, 2024 Switzerland's Swissmedic released an updated "Guidance on Export Certificates" and as part of its regulatory...

Sharan Murugan
Apr 7, 20242 min read


Swiss Medic: eCTD Guidance for Industry & Time limits for Authorisation Applications
Swissmedic, recently published updated guidance on "Guidance Industry eCTD" and "Guidance on Time limits for Authorisation Applications"....

Sharan Murugan
Mar 29, 20242 min read


Swissmedic Guidance: Navigating Variations, Product Information, and Electronic ICSRs through PV Gateway
Yesterday (06 February 2024) Switzerland's Swissmedic released updated guidance on "Electronic exchange of ICSRs through PV Gateway", and...

Sharan Murugan
Feb 7, 20242 min read


Swiss Medic: Biosimilar Authorisation Guidance
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance on (26 November 2023) the "Guidance document...

Sharan Murugan
Nov 26, 20232 min read

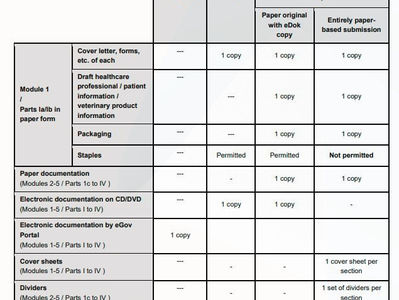
Switzerland's SwissMedic: Guidance on Formal Requirements
On 15 November 2023, Switzerland's Swissmedic released an updated guidance on "Formal Requirements" which serves as a resource for...

Sharan Murugan
Nov 15, 20232 min read


Switzerland's SwissMedic: Harmonisation of the SwissGMP/GDP Inspection System
Yesterday (20 July 2023) Switzerland's Swissmedic released an updated guidance on "Harmonisation of the SwissGMP/GDP Inspection System"...

Sharan Murugan
Jul 21, 20232 min read


Switzerland's SwissMedic: Guidance on Medicinal Product Name
Yesterday (07 July 2023) Switzerland's Swissmedic released an updated guidance on "Medicinal Product Name" that describes the...

Sharan Murugan
Jul 8, 20231 min read


Switzerland's SwissMedic: Guidance on Information of PSURs/PBRER submission
On 21 June 2023, Switzerland's Swissmedic released an updated guidance on "Information of PSURs/PBRER Submission" which describes the...

Sharan Murugan
Jun 25, 20231 min read


Switzerland's SwissMedic: Guidance on Authorisation Biosimilar
Yesterday (21 June 2023) Switzerland's Swissmedic released an updated guidance on "Authorisation Biosimilar" which specifies the...

Sharan Murugan
Jun 22, 20231 min read


SwissMedic Guidance: Orphan Drug, New Active Substance & Prior Notification
Swissmedic recently (02 June 2023) released multiple important guidelines that have captivated the audience and generated significant...

Sharan Murugan
Jun 4, 20232 min read


SwissMedic Guidance on Fast-Track Authorisation, Temporary Authorisation and Variations & Extensions
Last week Swissmedic, the Swiss Agency for Therapeutic Products updated and released 3 Important guidances and forms. 1. Guidance on...

Sharan Murugan
Jun 4, 20231 min read


Swiss Medic Guidance: Minimising the Risk of TSE and Authorisation of Radiopharmaceuticals
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance document on (26 May 2023) the "Guidance document...

Sharan Murugan
May 28, 20232 min read


Switzerland's Swissmedic: Med Dev Guidance on Export Certificates and Service Agreement
Yesterday (23 May 2023) Switzerland's Swissmedic released two updated guidances one is "Export Certificates" guidance and another one is...

Sharan Murugan
May 23, 20232 min read


Swiss Medic: Guidance on Transfer of Marketing Authorisation
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance document on (3 May 2023) the "Transfer of Marketing...

Sharan Murugan
May 7, 20231 min read
