Search

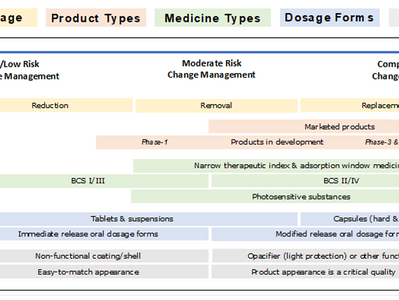
EMA’s Feedback: On Replacing Titanium Dioxide (TiO₂) in Medicinal Products: Critical Challenges, Limited Alternatives (Human & Veterneary)
The European Medicines Agency (EMA) submitted its updated report to the European Commission (EC) evaluating the feasibility of...

Sharan Murugan
Aug 6, 20253 min read


USFDA Guidance: Size, Shape, & Other Physical Attributes of Generic Tablets and Capsules
Earlier today (03-October-2022) USFDA's Center for Drug Evaluation and Research released an important CMC topic and final guidance "...

Sharan Murugan
Oct 3, 20221 min read
