Search


USFDA: CDER Establishes New Center for Real-World Evidence Innovation (CCRI)
The U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) has unveiled an innovative initiative—the CDER...

Sharan Murugan
Dec 15, 20242 min read


USFDA Guidance: Standardized Format for Electronic Submission of NDA and BLA Content for Bioresearch Monitoring (BIMO) Inspections
The United States Food and Drug Administration (USFDA) has released detailed guidance" Standardized Format for Electronic Submission of...

Sharan Murugan
Dec 9, 20242 min read


USFDA Draft Guidance: Accelerated Approval – Expedited Program for Serious Conditions
The United States Food and Drug Administration (USFDA) has released (05 December, 2024) the draft guidance " Expedited Program for...

Sharan Murugan
Dec 9, 20242 min read


USFDA Final Guidance: Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions
The US Food and Drug Administration (USFDA) has issued its final guidance on " Marketing Submission Recommendations for a Predetermined...

Sharan Murugan
Dec 9, 20242 min read


USFDA Guidance: Enhancing Communication and Guidance Development Practices
The US Food and Drug Administration (USFDA) has released two insightful reports aimed at improving its internal practices and...

Sharan Murugan
Dec 4, 20242 min read


USFDA MD Guidance: Enhancing Safety and Efficiency in Orthopedic Devices and Sterilization Processes
The USFDA Center for Devices and Radiological Health has recently issued three key final guidance documents focusing on orthopedic...

Sharan Murugan
Nov 27, 20242 min read

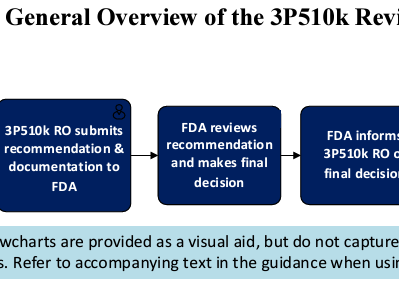
USFDA Guidance: 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review
The 510(k) Third Party Review Program and Third Party Emergency Use Authorization (EUA) Review are pivotal initiatives by the USFDA to...

Sharan Murugan
Nov 20, 20242 min read


USFDA Guidance: Nonclinical Safety Assessment of Oligonucleotide-Based Therapeutics
This USFDA draft guidance" Nonclinical Safety Assessment of Oligonucleotide-Based Therapeutic s" , issued on 15 November 2024, offers...

Sharan Murugan
Nov 16, 20242 min read


USFDA Guidance: Study Data Technical Conformance Guide - Technical Specifications
The Study Data Technical Conformance Guide (SDTCG) from the USFDA provides a framework to help sponsors submit standardized study data...

Sharan Murugan
Nov 9, 20242 min read


USFDA Guidance: M13A Bioequivalence for Immediate-Release Solid Oral Dosage Forms
Recently 30th October, 2025 the U.S. Food and Drug Administration (FDA) issued guidance titled " M13A: Bioequivalence for...

Sharan Murugan
Nov 1, 20242 min read


US FDA Draft Guidance: Drug Interaction Information in Human Prescription Drug and Biological Product Labeling
The US FDA released a draft guidance on 21st October 2024 outlining the recommendations for " Drug Interaction Information in Human...

Sharan Murugan
Oct 22, 20242 min read


USFDA Guidance: Core Patient-Reported Outcomes in Cancer Clinical Trials & Considerations for Long-Term Clinical Neurodevelopmental Safety Studies in Neonatal Product Development
The U.S. Food and Drug Administration (USFDA) yesterday (17 October, 2024) has issued two comprehensive Q&A guidance titled " Core...

Sharan Murugan
Oct 17, 20242 min read


USFDA Guidance : Electronic Systems, Electronic Records, and Electronic Signatures in Clinical Investigations: Questions and Answers
The U.S. Food and Drug Administration (USFDA) has issued comprehensive Q&A guidance titled " Electronic Systems, Electronic Records, and...

Sharan Murugan
Oct 2, 20242 min read


USFDA Medical Device: Guidance on Dental Products (Dental Impression Materials, Dental Ceramics, Dental Cements and Air Powered Dental Handpieces and Air Motors)
USFDA's Center for Devices and Radiological Health has released four final guidances about dental products, such as Dental Impression...

Sharan Murugan
Sep 28, 20242 min read


USFDA Guidance: An Acceptable Circular of Information for the Use of Human Blood and Blood Components
The USFDA's Center for Biologics Evaluation and Research (CBER) has released the final guidance document , titled “ An Acceptable...

Sharan Murugan
Sep 25, 20242 min read


USFDA Guidance: Providing Regulatory Submissions in Electronic Format Using eCTD Specifications
This Friday (13 Septmeber, 2024) the USFDA's Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research...

Sharan Murugan
Sep 15, 20242 min read


USFDA Guidance: Amendments to Abbreviated New Drug Applications (ANDAs) Under GDUFA
FDA's Center for Drug Evaluation and Research revised the final guidance last week titled " ANDA Submissions | Amendments to Abbreviated...

Sharan Murugan
Sep 15, 20242 min read


USFDA Guidance: Appeal Options for Mammography Facilities
The USFDA (U.S. Food and Drug Administration) Center for Devices and Radiological Health provides detailed final guidance " Appeal...

Sharan Murugan
Sep 15, 20242 min read


USFDA Guidance: Incorporating Voluntary Patient Preference Information Over the Total Product Life Cycle
USFDA's Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER) released a draft...

Sharan Murugan
Sep 6, 20242 min read


USFDA Guidance: Control of Nitrosamine Impurities in Human Drugs
Earlier today (04 September, 2024) U.S. Food and Drug Administration's Center for Drug Evaluation and Research released the second...

Sharan Murugan
Sep 4, 20242 min read
