Saudi Arabia (SFDA) New Guidance: On Digital Health Products
- Sharan Murugan

- Sep 4, 2025
- 3 min read
Digital health is no longer just an emerging trend—it is rapidly transforming healthcare delivery, patient engagement, and medical innovation worldwide. From mobile health apps to artificial intelligence-enabled devices, digital solutions are increasingly used to monitor, diagnose, and even treat medical conditions. Recognizing this fast growth, the Saudi Food and Drug Authority (SFDA) has issued the Guidance on Digital Health Products to clarify how different digital health technologies are regulated in the Kingdom of Saudi Arabia (KSA).
Defining Digital Health Products
According to SFDA, a digital health product refers to the use of Information and Communication Technologies (ICT) in healthcare, intended to improve outcomes or enhance delivery of medical services.
Whether a product qualifies as a medical device depends on its intended use, as stated by the manufacturer in labeling, instructions, or technical documentation. If the intended purpose is related to diagnosis, prevention, monitoring, treatment, or management of medical conditions, it falls under the Medical Devices Law of Saudi Arabia and must be regulated accordingly
Purpose and Scope of the Guidance
The SFDA guidance aims to:
Define which types of digital health products qualify as medical devices.
Provide clarity to manufacturers, software developers, and healthcare providers seeking Medical Device Marketing Authorization (MDMA) in Saudi Arabia.
Outline categories of digital health products under regulatory oversight versus those considered general wellness devices.
It covers major areas of digital health, including:
Software as a Medical Device (SaMD)
Mobile Health Applications (mHealth apps)
Digital Therapeutics (DTx)
Health Information Technology (HIT)
Telemedicine
Wearable Devices
Virtual Reality & Augmented Reality (VR/AR)
Artificial Intelligence and Machine Learning (AI/ML)
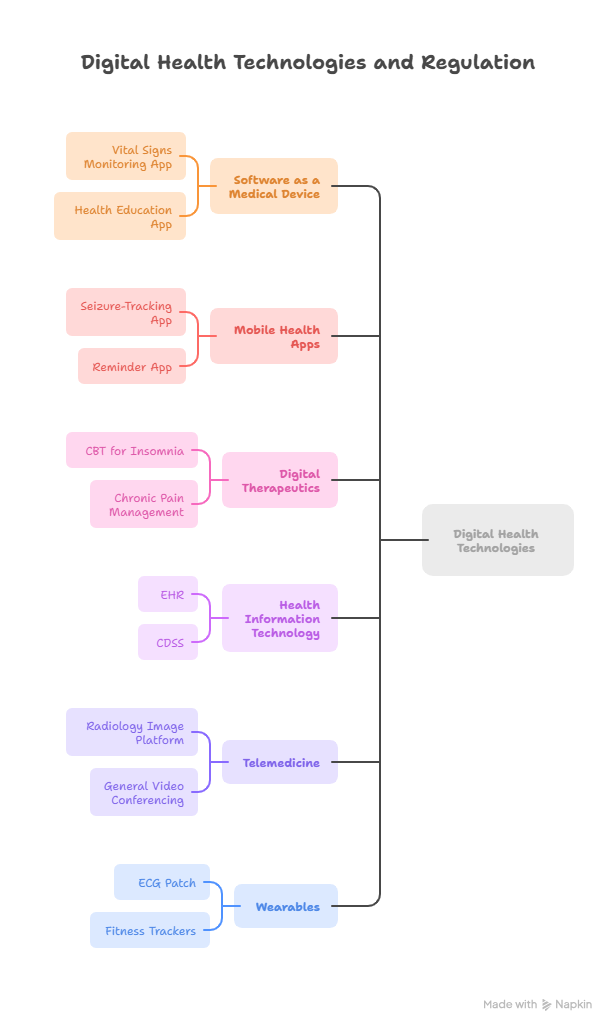
Key Categories of Digital Health Products
1. Software as a Medical Device (SaMD)
Defined by the IMDRF as “software intended to be used for one or more medical purposes that performs these purposes without being part of a hardware medical device.”
Examples that qualify: software monitoring vital signs and alerting patients or clinicians of abnormalities.
Not SaMD: educational apps or communication platforms without diagnostic or therapeutic purpose.
2. Mobile Health Applications (mHealth Apps)
Apps designed for smartphones, tablets, or wearables, supporting healthcare functions.
Qualify as medical devices: seizure tracking apps, apps using sensors for glucose monitoring, or image-processing apps for diagnostics.
Do not qualify: medication reminder apps or nutrition trackers.
3. Digital Therapeutics (DTx)
Evidence-based software interventions designed to treat or manage diseases.
Examples: apps delivering cognitive behavioral therapy for insomnia, or programs providing personalized exercise for chronic pain.
Always regulated as SaMD, requiring clinical validation.
4. Health Information Technology (HIT)
Systems like Electronic Health Records (EHRs), Clinical Decision Support Systems (CDSS), and e-prescribing software.
Regulated only when they analyze or interpret data for medical purposes.
Purely administrative systems (e.g., appointment scheduling or billing) are not medical devices.
5. Telemedicine
Use of digital communication to deliver healthcare remotely.
Regulated as medical devices when used for diagnosis, monitoring, or treatment (e.g., radiology image platforms).
Not regulated: general-purpose video conferencing tools.
6. Wearable Devices
Devices worn on the body to monitor health.
Examples of regulated devices: smartwatches detecting irregular heart rhythms, ECG patches.
General wellness devices: step counters or basic fitness trackers without diagnostic claims.
7. Virtual Reality & Augmented Reality (VR/AR)
Immersive technologies with growing medical applications.
Qualify as medical devices when used in surgery planning, pain therapy, or rehabilitation.
Not regulated: VR/AR used only for training or education.
8. Artificial Intelligence & Machine Learning (AI/ML)
AI/ML introduces unique complexities requiring special regulatory oversight. SFDA requires:
Validation & Verification (V&V) with high-quality datasets.
Transparency & Explainability of algorithms.
Risk management covering bias, cybersecurity, and unintended outcomes.
Lifecycle oversight, including post-market monitoring and algorithm updates.
General Wellness Devices
Products that make wellness claims without specific medical purposes, such as:
Apps promoting exercise, meditation, or nutrition.
Devices claiming to support “healthy living” without diagnosis or treatment.
If a wellness product is marketed or used for medical purposes, SFDA may reclassify it as a regulated medical device. Manufacturers must also label such devices clearly in Arabic and English as “not intended for medical purposes.”
For manufacturers and developers, the message is clear: carefully define the intended use of your digital health product, and prepare to meet regulatory requirements if it crosses into the realm of medical devices.
👉 For more details, refer to the official SFDA Guidance on Digital Health Products.



Comments