Search


Saudi Arabia (SFDA) New Guidance: On Digital Health Products
Digital health is no longer just an emerging trend—it is rapidly transforming healthcare delivery, patient engagement, and medical...

Sharan Murugan
Sep 4, 20253 min read


South Africa: Renewal of Medicines Certificate of Registration Framework
On 26th August, 2025 the South African Health Products Regulatory Authority (SAHPRA) has introduced a structured framework for the...

Sharan Murugan
Aug 28, 20252 min read


TGA Guidance: GMP Update for Medicinal Products in Australia: Transitioning to PIC/S Guide PE009-17
Good Manufacturing Practice (GMP) serves as the backbone of pharmaceutical quality assurance. It ensures that medicinal products are...

Sharan Murugan
Aug 28, 20252 min read


Swissmedic Guidance: Clinical Trials with Medicinal Products, Submission Process and FAQs
Clinical trials are the cornerstone of developing safe and effective medicines. In Switzerland, the regulatory authority...

Sharan Murugan
Aug 28, 20252 min read


SAHPRA Communication: Regulatory Requirements for AI/ML-Enabled Medical Devices in South Africa
Artificial Intelligence (AI) and Machine Learning (ML) are transforming healthcare globally, offering powerful tools for diagnosis,...

Sharan Murugan
Aug 23, 20252 min read


Swissmedic Guidance: Submission Process for Clinical Trials with Medicinal Products
On 18 August 2025, Swissmedic published Version 2.3 of its guidance on the " Submission Process for Clinical Trials with Medicinal...

Sharan Murugan
Aug 23, 20252 min read


USFDA Med Dev Guidance: Animal Studies for Dental Bone Grafting Material Devices in 510(k) Submissions
On 22 August 2025 , the U.S. Food and Drug Administration (FDA) released a comprehensive guidance document titled “ Animal Studies for...

Sharan Murugan
Aug 23, 20252 min read


UK MHRA Guidance: Applying for Clinical Trial Authorisation (CTA)
On 22 August 2025 , the Medicines and Healthcare products Regulatory Agency (MHRA) published an updated version of its guidance “...

Sharan Murugan
Aug 23, 20252 min read


USFDA Draft Guidance: Radiopharmaceutical Dosage Optimization and Overall Survival Assesment in Oncology Clinical Trials
On 18th August 2025, the U.S. Food and Drug Administration (FDA) recently published two important draft guidance documents that aim to...

Sharan Murugan
Aug 19, 20252 min read


USFDA Guidance: Predetermined Change Control Plans (PCCPs) for AI/ML Medical Devices
Artificial Intelligence and Machine Learning (AI/ML) are increasingly used in medical devices—from diagnostic imaging software to digital...

Sharan Murugan
Aug 19, 20252 min read


UK MHRA Guidance: How to Cancel a Medicine’s Marketing Authorisation or Other Licence
In the pharmaceutical lifecycle, there are times when a marketing authorisation holder (MAH) needs to cancel a medicine’s marketing...

Sharan Murugan
Aug 19, 20252 min read

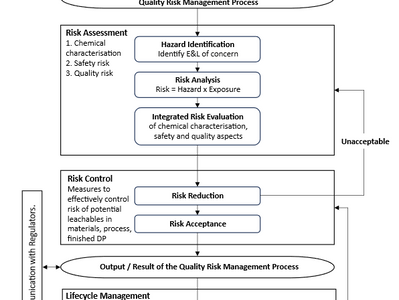
ICH Q3E – Impurities: Extractables and Leachables for Pharmaceuticals and Biologics
On 1 August 2025, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has...

Sharan Murugan
Aug 13, 20252 min read


India CDSCO Launches: New Online Dual Use NOC System via SUGAM Portal
The Central Drugs Standard Control Organization (CDSCO), under the Ministry of Health and Family Welfare, has released a notice a...

Sharan Murugan
Aug 13, 20252 min read


Malaysia–China Medical Device Regulatory Reliance Programme: Pilot Phase I (30 July – 30 September 2025)
The Medical Device Authority (MDA) of Malaysia has officially launched the Malaysia–China Medical Device Regulatory Reliance Programme ,...

Sharan Murugan
Aug 13, 20252 min read


EMA Publishes First EU eCTD v4.0 Validation Criteria and Updated Controlled Vocabularies
In 1st & 8th August 2025, the European Medicines Agency (EMA) announced two major milestones in the transition to electronic Common...

Sharan Murugan
Aug 10, 20252 min read


European Commission Insights: Study on the Deployment of Artificial Intelligence in Healthcare – 2025
On 8 August 2025, the European Commission released an in-depth study on the " Deployment of artificial intelligence (AI) in healthcare...

Sharan Murugan
Aug 10, 20252 min read


EMA Procedural Advice: Paediatric Applications – A Comprehensive Guide for Applicants
On 8 August 2025 , the European Medicines Agency (EMA) published the latest revision (Rev. 14) of its "Procedural Advice on Paediatric...

Sharan Murugan
Aug 10, 20252 min read

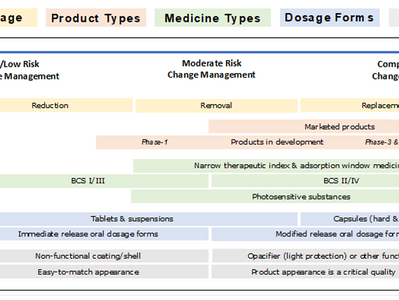
EMA’s Feedback: On Replacing Titanium Dioxide (TiO₂) in Medicinal Products: Critical Challenges, Limited Alternatives (Human & Veterneary)
The European Medicines Agency (EMA) submitted its updated report to the European Commission (EC) evaluating the feasibility of...

Sharan Murugan
Aug 6, 20253 min read

UK MHRA Med Dev Guidance: Clinical Investigations for Medical Devices - What Sponsors Need to Know in 2025
Medical devices, whether diagnostic, therapeutic, or assistive, undergo rigorous scrutiny before entering the UK market. One of the most...

Sharan Murugan
Aug 6, 20253 min read


UK MHRA Guidance: Adverse Event Reporting in Digital Mental Health Technologies (DMHTs)
The proliferation of Digital Mental Health Technologies (DMHTs) —ranging from self-help apps and online therapy services to artificial...

Sharan Murugan
Aug 2, 20252 min read
