Search


USFDA Guidance: UDI Requirements for Combination Products and Cybersecurity for Medical Devices
In June 2025, the FDA released two impactful draft guidances that significantly affect medical device and combination product...

Sharan Murugan
Jun 293 min read

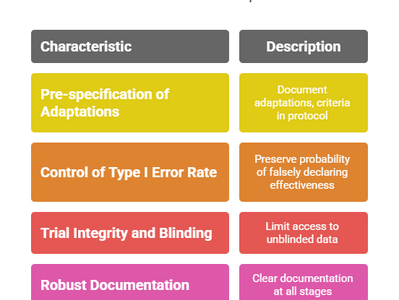
ICH E20 Draft Guideline: Understanding Adaptive Clinical Trials in Focus (2025)
The International Council for Harmonisation (ICH) has taken a major step toward modernizing clinical trial designs with the release of the...

Sharan Murugan
Jun 283 min read


UK MHRA Guidances: 9 important Guidances on Clinical Trials Safety, Approvals, Labelling, and More
Clinical trials are the cornerstone of modern medicine, ensuring that new treatments are safe, effective, and suitable for public use....

Sharan Murugan
Jun 283 min read


ICH & USFDA Draft Guidance: Q1 Stability Testing: The Gold Standard for Drug Shelf Life and Quality
The stability of drug substances and drug products is a cornerstone of pharmaceutical quality, ensuring that medicines remain safe,...

Sharan Murugan
Jun 233 min read


EMA Guidance: EMA’s Implementation of ISO IDMP Standards Through Products Management Services (PMS)
To modernize and harmonize the management of medicinal product information, the European Medicines Agency (EMA) is actively implementing...

Sharan Murugan
Jun 232 min read


USFDA Guidance: Post-Warning Letter Meetings Under GDUFA III: A Regulatory Pathway Toward Compliance
In the evolving regulatory landscape of generic drug manufacturing, compliance and transparency are more crucial than ever. The FDA’s...

Sharan Murugan
Jun 182 min read


EMA Guidance: A Guide to the HMA-EMA Real-World Evidence Catalogues
As the role of real-world data (RWD) and real-world evidence (RWE) grows in drug development, regulatory decision-making, and...

Sharan Murugan
Jun 183 min read


UK MHRA Guidance: Get Scientific Advice from MHRA- Comprehensive Guide
Navigating the regulatory landscape for medicines in the UK can be complex, especially as requirements evolve and innovation accelerates....

Sharan Murugan
Jun 183 min read


UK MHRA Guidance: Electronic Common Technical Document (eCTD) Submissions Update
The Medicines and Healthcare products Regulatory Agency (MHRA) published a key update on 17 June 2025 , outlining revised requirements...

Sharan Murugan
Jun 182 min read


USFDA Guidance: ANDAs: Pre-Submission Facility Correspondence Related to Prioritized Generic Drug Submissions
The U.S. Food and Drug Administration (FDA) has released an updated guidance " ANDAs: Pre-Submission Facility Correspondence Related to...

Sharan Murugan
Jun 132 min read


EMA’s User Guide: HMA-EMA Catalogues of Real-World Data Sources and Studies
The integration of real-world data (RWD) into regulatory decision-making is transforming the landscape of pharmaceutical development and...

Sharan Murugan
Jun 132 min read


UK MHRA Guidance: GLP-1 Medicines for Weight Loss and Diabetes: What You Need to Know
In recent years, GLP-1 receptor agonists have gained widespread attention for their dual role in type 2 diabetes management and weight...

Sharan Murugan
Jun 132 min read


UK MHRA's Guidance: UK’s Decentralised Manufacture Framework: A Holistic Overview
In the evolving landscape of pharmaceutical manufacturing, Decentralised Manufacture has emerged as a pivotal approach, especially in...

Sharan Murugan
Jun 133 min read


SAHPRA’s Biological Medicines Amendment Guideline - A Critical Update for Biologic Product Sponsors
The South African Health Products Regulatory Authority (SAHPRA) has released the sixth version of its Biological Medicines Amendment...

Sharan Murugan
Jun 72 min read


Swissmedic’s Clinical Trial Guidance Suite: Everything Sponsors Need to Know
On June 2, 2025 , Swissmedic published a harmonised suite of guidance documents to standardise, streamline, and digitalise the clinical...

Sharan Murugan
Jun 72 min read


USFDA Guidance: M11 Technical Specification & Template: Clinical Electronic Structured Harmonised Protocol (CeSHarP)
In a major step toward global harmonisation of clinical trial processes, the U.S. Food and Drug Administration (FDA) and the...

Sharan Murugan
Jun 72 min read


EMA Guidance: Core SmPC Guideline for Subcutaneous and Intramuscular Immunoglobulins: What Manufacturers Need to Know
Human normal immunoglobulin (IgG) products administered via the subcutaneous (SCIg) or intramuscular (IMIg) route are essential...

Sharan Murugan
Jun 52 min read


Meet ELSA: USFDA Launches Agency-Wide AI Tool to Optimize Performance
What if the agonizing wait for drug approvals could be slashed from days to minutes? Imagine a future where breakthrough therapies reach...

Sharan Murugan
Jun 22 min read


USFDA’s Draft Guidance: Bioequivalence Biowaivers for Additional Strengths of Immediate-Release Oral Drugs
Developing drug products across multiple strengths is a common strategy in pharmaceutical formulation, allowing dose flexibility and...

Sharan Murugan
May 302 min read


USFDA Draft Guidance: Replacing Color Additives in Approved or Marketed Drug Products
A color additive is any dye, pigment, or substance that imparts color to a drug. Only color additives listed in FDA regulations are...

Sharan Murugan
May 302 min read
