Search


MHRA Updates Comprehensive Guidance Framework for Clinical Trials in the UK (October 2025)
The UK’s Medicines and Healthcare products Regulatory Agency ( MHRA ) has issued a coordinated update to its suite of clinical trial...

Sharan Murugan
Oct 5, 20253 min read


EMA Guidance: European Commission’s (EC) New Variations Guidelines
On 22 September 2025 , the European Commission published new variations guidelines in the Official Journal of the European Union. These...

Sharan Murugan
Sep 22, 20252 min read


USFDA Draft Guidance on Safety Labeling Changes: Streamlining Section 505(o)(4) Implementation
On September 19, 2025, the U.S. Food and Drug Administration (FDA) released its revised draft guidance, “ Safety Labeling...

Sharan Murugan
Sep 21, 20253 min read

USFDA Guidance: GERD-Related Drug Development: A Comprehensive Update
The U.S. Food and Drug Administration (FDA) released three important draft guidances on September 11, 2025, providing updated...

Sharan Murugan
Sep 21, 20252 min read


USFDA Guidance: Alternative Tools for Facility Assessments in Pending Applications
The U.S. Food and Drug Administration (FDA) has issued a final guidance on 11th September 2025 titled “ Alternative Tools: Assessing Drug...

Sharan Murugan
Sep 14, 20252 min read


USFDA Guidances: Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment, Other Quality-Related Considerations & Classification Categories for Certain Supplements
In 08 September 2025, the U.S. Food and Drug Administration (FDA) issued two final guidances addressing critical aspects of biosimilar...

Sharan Murugan
Sep 9, 20252 min read


Swissmedic Guidances: Strengthening Regulatory Clarity for Medicinal Products
On 1 September 2025 , Swissmedic published a set of updated guidance documents that refine requirements for medicinal product...

Sharan Murugan
Sep 7, 20252 min read


UK MHRA Guidance: Navigating New MHRA Payments & Fees guidance
The Medicines and Healthcare products Regulatory Agency (MHRA) plays a pivotal role in regulating medicines, medical devices, and related...

Sharan Murugan
Sep 4, 20253 min read


South Africa: Renewal of Medicines Certificate of Registration Framework
On 26th August, 2025 the South African Health Products Regulatory Authority (SAHPRA) has introduced a structured framework for the...

Sharan Murugan
Aug 28, 20252 min read


TGA Guidance: GMP Update for Medicinal Products in Australia: Transitioning to PIC/S Guide PE009-17
Good Manufacturing Practice (GMP) serves as the backbone of pharmaceutical quality assurance. It ensures that medicinal products are...

Sharan Murugan
Aug 28, 20252 min read


Swissmedic Guidance: Clinical Trials with Medicinal Products, Submission Process and FAQs
Clinical trials are the cornerstone of developing safe and effective medicines. In Switzerland, the regulatory authority...

Sharan Murugan
Aug 28, 20252 min read


Swissmedic Guidance: Submission Process for Clinical Trials with Medicinal Products
On 18 August 2025, Swissmedic published Version 2.3 of its guidance on the " Submission Process for Clinical Trials with Medicinal...

Sharan Murugan
Aug 23, 20252 min read


UK MHRA Guidance: Applying for Clinical Trial Authorisation (CTA)
On 22 August 2025 , the Medicines and Healthcare products Regulatory Agency (MHRA) published an updated version of its guidance “...

Sharan Murugan
Aug 23, 20252 min read


USFDA Draft Guidance: Radiopharmaceutical Dosage Optimization and Overall Survival Assesment in Oncology Clinical Trials
On 18th August 2025, the U.S. Food and Drug Administration (FDA) recently published two important draft guidance documents that aim to...

Sharan Murugan
Aug 19, 20252 min read


UK MHRA Guidance: How to Cancel a Medicine’s Marketing Authorisation or Other Licence
In the pharmaceutical lifecycle, there are times when a marketing authorisation holder (MAH) needs to cancel a medicine’s marketing...

Sharan Murugan
Aug 19, 20252 min read

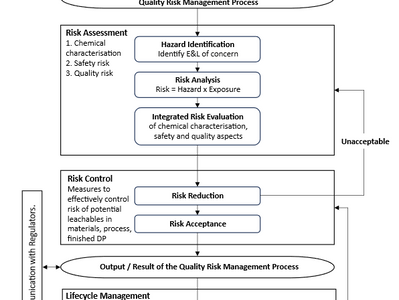
ICH Q3E – Impurities: Extractables and Leachables for Pharmaceuticals and Biologics
On 1 August 2025, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has...

Sharan Murugan
Aug 13, 20252 min read


EMA Publishes First EU eCTD v4.0 Validation Criteria and Updated Controlled Vocabularies
In 1st & 8th August 2025, the European Medicines Agency (EMA) announced two major milestones in the transition to electronic Common...

Sharan Murugan
Aug 10, 20252 min read


European Commission Insights: Study on the Deployment of Artificial Intelligence in Healthcare – 2025
On 8 August 2025, the European Commission released an in-depth study on the " Deployment of artificial intelligence (AI) in healthcare...

Sharan Murugan
Aug 10, 20252 min read


EMA Procedural Advice: Paediatric Applications – A Comprehensive Guide for Applicants
On 8 August 2025 , the European Medicines Agency (EMA) published the latest revision (Rev. 14) of its "Procedural Advice on Paediatric...

Sharan Murugan
Aug 10, 20252 min read

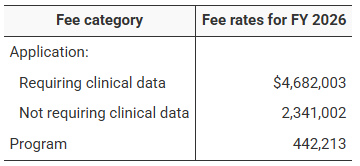
USFDA FDA User Fee Rates for FY 2026: A Comparative Overview for Prescription Drugs, Medical Devices, Generics, and Outsourcing Facilities
The U.S. Food and Drug Administration (FDA) has announced On 30 July 2025 , the official user fee rates across its major healthcare...

Sharan Murugan
Aug 2, 20252 min read
