Search


South Africa: Renewal of Medicines Certificate of Registration Framework
On 26th August, 2025 the South African Health Products Regulatory Authority (SAHPRA) has introduced a structured framework for the...

Sharan Murugan
Aug 28, 20252 min read


TGA Guidance: GMP Update for Medicinal Products in Australia: Transitioning to PIC/S Guide PE009-17
Good Manufacturing Practice (GMP) serves as the backbone of pharmaceutical quality assurance. It ensures that medicinal products are...

Sharan Murugan
Aug 28, 20252 min read


Swissmedic Guidance: Clinical Trials with Medicinal Products, Submission Process and FAQs
Clinical trials are the cornerstone of developing safe and effective medicines. In Switzerland, the regulatory authority...

Sharan Murugan
Aug 28, 20252 min read


Swissmedic Guidance: Submission Process for Clinical Trials with Medicinal Products
On 18 August 2025, Swissmedic published Version 2.3 of its guidance on the " Submission Process for Clinical Trials with Medicinal...

Sharan Murugan
Aug 23, 20252 min read


UK MHRA Guidance: Applying for Clinical Trial Authorisation (CTA)
On 22 August 2025 , the Medicines and Healthcare products Regulatory Agency (MHRA) published an updated version of its guidance “...

Sharan Murugan
Aug 23, 20252 min read


USFDA Draft Guidance: Radiopharmaceutical Dosage Optimization and Overall Survival Assesment in Oncology Clinical Trials
On 18th August 2025, the U.S. Food and Drug Administration (FDA) recently published two important draft guidance documents that aim to...

Sharan Murugan
Aug 19, 20252 min read


UK MHRA Guidance: How to Cancel a Medicine’s Marketing Authorisation or Other Licence
In the pharmaceutical lifecycle, there are times when a marketing authorisation holder (MAH) needs to cancel a medicine’s marketing...

Sharan Murugan
Aug 19, 20252 min read

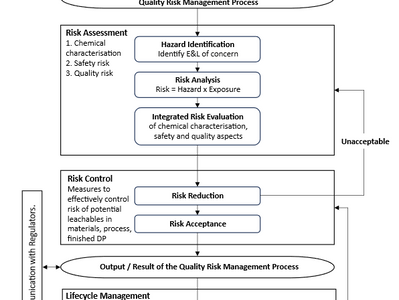
ICH Q3E – Impurities: Extractables and Leachables for Pharmaceuticals and Biologics
On 1 August 2025, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has...

Sharan Murugan
Aug 13, 20252 min read


EMA Publishes First EU eCTD v4.0 Validation Criteria and Updated Controlled Vocabularies
In 1st & 8th August 2025, the European Medicines Agency (EMA) announced two major milestones in the transition to electronic Common...

Sharan Murugan
Aug 10, 20252 min read


European Commission Insights: Study on the Deployment of Artificial Intelligence in Healthcare – 2025
On 8 August 2025, the European Commission released an in-depth study on the " Deployment of artificial intelligence (AI) in healthcare...

Sharan Murugan
Aug 10, 20252 min read


EMA Procedural Advice: Paediatric Applications – A Comprehensive Guide for Applicants
On 8 August 2025 , the European Medicines Agency (EMA) published the latest revision (Rev. 14) of its "Procedural Advice on Paediatric...

Sharan Murugan
Aug 10, 20252 min read

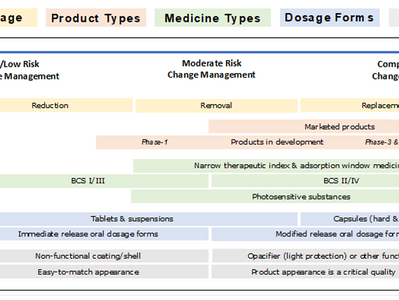
EMA’s Feedback: On Replacing Titanium Dioxide (TiO₂) in Medicinal Products: Critical Challenges, Limited Alternatives (Human & Veterneary)
The European Medicines Agency (EMA) submitted its updated report to the European Commission (EC) evaluating the feasibility of...

Sharan Murugan
Aug 7, 20253 min read

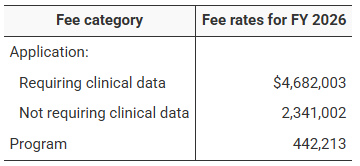
USFDA FDA User Fee Rates for FY 2026: A Comparative Overview for Prescription Drugs, Medical Devices, Generics, and Outsourcing Facilities
The U.S. Food and Drug Administration (FDA) has announced On 30 July 2025 , the official user fee rates across its major healthcare...

Sharan Murugan
Aug 2, 20252 min read


EMA Guidance: Procedural Advice on Paediatric Applications
On 25 July 2025 , the European Medicines Agency (EMA) published the latest guidance " Procedural Advice on Paediatric Applications " ....

Sharan Murugan
Jul 31, 20252 min read

ICH -E21 (USFDA) Guidance: Including Pregnant and Breastfeeding Women in Clinical Trials
In May 2025, the FDA, as part of the International Council for Harmonisation (ICH), endorsed and released for consultation the draft E21...

Sharan Murugan
Jul 27, 20253 min read


EMA Guidance: Good Pharmacovigilance Practices (GVP) — Introductory cover note with new Addendum II and GVP Module VI Addendum II – Masking of Personal Data
The European Medicines Agency (EMA) continuously updates its Good Pharmacovigilance Practices (GVP) guidelines to enhance patient privacy...

Sharan Murugan
Jul 27, 20253 min read


Malaysia’s NPRA Guidance: Drug Registration Guidance, What’s Inside?
On 22nd July 2025, Malaysia’s National Pharmaceutical Regulatory Agency (NPRA) released the Third Edition, Tenth Revision of the " Drug...

Sharan Murugan
Jul 23, 20252 min read


USFDA's: FDA’s Commissioner’s National Priority Voucher (CNPV) Pilot Program to Accelerate Life-Changing Medicines
The U.S. Food and Drug Administration (FDA) has launched the " Commissioner’s National Priority Voucher (CNPV) Pilot Program " a...

Sharan Murugan
Jul 23, 20252 min read

EMA Guidance: Requirements for Demonstrating Therapeutic equivalence between Orally Inhaled Products (OIP) and Chronic Obstructive Pulmonary Disease (COPD)
On 14 July 2025 , the European Medicines Agency (EMA) published the final revised guideline titled " Guideline on the requirements for...

Sharan Murugan
Jul 23, 20252 min read


India CDSCO Guidance: Subject Expert Committee (SEC), What Pharma and Clinical Trial Teams Need to Know
The Central Drugs Standard Control Organization (CDSCO) under India’s Ministry of Health & Family Welfare has released a detailed...

Sharan Murugan
Jul 23, 20252 min read
