Search


SAHPRA Communication: Regulatory Requirements for AI/ML-Enabled Medical Devices in South Africa
Artificial Intelligence (AI) and Machine Learning (ML) are transforming healthcare globally, offering powerful tools for diagnosis,...

Sharan Murugan
Aug 23, 20252 min read


USFDA Guidance: Predetermined Change Control Plans (PCCPs) for AI/ML Medical Devices
Artificial Intelligence and Machine Learning (AI/ML) are increasingly used in medical devices—from diagnostic imaging software to digital...

Sharan Murugan
Aug 19, 20252 min read


European Commission Insights: Study on the Deployment of Artificial Intelligence in Healthcare – 2025
On 8 August 2025, the European Commission released an in-depth study on the " Deployment of artificial intelligence (AI) in healthcare...

Sharan Murugan
Aug 10, 20252 min read

European Commission: Guidelines on the Scope of the Obligations for General-Purpose AI Models established by AI Act
On 18 July 2025, the European Commission published detailed Guidelines on the scope of the obligations for providers of general-purpose...

Sharan Murugan
Jul 19, 20253 min read

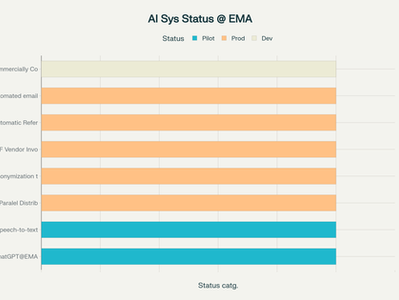
EMA’s AI Journey: The Rise of Artificial Intelligence in Medicines Regulation
In a world where artificial intelligence (AI) is moving rapidly from theory to practice, regulators are tasked with keeping pace—not only...

Sharan Murugan
Jul 19, 20253 min read


EMA NDSG Work Plan: Inside the Network Data Steering Group Workplan 2025–2028
In an era where data drives decisions and innovation defines progress, the European Medicines Agency (EMA) and Heads of Medicines...

Sharan Murugan
Jul 2, 20253 min read


UK MHRA News: "AI Airlock" Pioneering Safe Healthcare Innovation in the UK
The UK’s Medicines & Healthcare products Regulatory Agency (MHRA) has launched Phase 2 of its AI Airlock programme , supported by a...

Sharan Murugan
Jun 23, 20252 min read


Meet ELSA: USFDA Launches Agency-Wide AI Tool to Optimize Performance
What if the agonizing wait for drug approvals could be slashed from days to minutes? Imagine a future where breakthrough therapies reach...

Sharan Murugan
Jun 2, 20252 min read


EMA Network Data Steering Group workplan 2025-2028: Leveraging Data and AI for Enhanced Medicine Regulation
In the evolving landscape of medicines regulation, data and artificial intelligence (AI) have become pivotal tools to enhance public and...

Sharan Murugan
May 11, 20253 min read


Announcing Veeva AI: A Bold Leap Toward Intelligent Automation in Life Sciences
The world of pharma and life sciences is abuzz with a major announcement: Veeva Systems has just unveiled Veeva AI , a groundbreaking...

Sharan Murugan
Apr 29, 20253 min read


EMA's New AI Work Plan: Shaping the Future of Medicines Regulation
The European Medicines Agency (EMA) has unveiled a comprehensive Artificial Intelligence (AI) Work Plan to guide the integration of AI...

Sharan Murugan
Mar 31, 20252 min read


USFDA Guidance: Artificial Intelligence-Enabled Device Software Functions – Lifecycle Management and Marketing Submission Recommendations
The U.S. Food and Drug Administration (FDA) has issued a draft guidance document titled “ Artificial Intelligence-Enabled Device Software...

Sharan Murugan
Jan 7, 20252 min read


USFDA Draft Guidance: Considerations for the Use of Artificial Intelligence to Support Regulatory Decision
The U.S. Food and Drug Administration (FDA) released a draft guidance earlier today (07 January, 2025) outlining " Considerations for...

Sharan Murugan
Jan 7, 20252 min read


USFDA Final Guidance: Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions
The US Food and Drug Administration (USFDA) has issued its final guidance on " Marketing Submission Recommendations for a Predetermined...

Sharan Murugan
Dec 9, 20242 min read


UK MHRA Press Release Trials Innovative AI Technologies in Regulatory Pilot Scheme
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) has launched (4th December 2024) a groundbreaking pilot scheme "...

Sharan Murugan
Dec 4, 20242 min read


EMA: Harnessing AI in Medicines Regulation: The Role of Large Language Models (LLMs)
The advancement of Artificial Intelligence (AI) is transforming many industries, and the pharmaceutical and regulatory sectors are no...

Sharan Murugan
Sep 15, 20242 min read


New EU Regulations on AI in Medical Devices: Key Insights and Implications
The European Union has published the finalized text of the Artificial Intelligence Act (AIA) which is a significant step forward in...

Sharan Murugan
Jul 15, 20242 min read


IMDRF Guidance: Good Machine Learning Practice for Medical Device Development: Guiding Principles
The International Medical Device Regulators Forum (IMDRF) has published a draft guidance (01 July, 2024) "Good machine learning practice...

Sharan Murugan
Jul 3, 20242 min read


UK MHRA: "AI Airlock" The Regulatory Sandbox for AIaMD
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has introduced (09 May, 2024) the “AI Airlock - The Regulatory Sandbox...

Sharan Murugan
May 18, 20242 min read


UK MHRA: Guidance on Software and Artificial Intelligence (AI) as a Medical Device
Last Friday (03 May 2024) UK's Medicines and Healthcare Products Regulatory Agency (MHRA) released updated guidance on "Software and...

Sharan Murugan
May 6, 20242 min read
