Search


USFDA Q&A: Promotional Labeling and Advertising Considerations for Prescription Biological Reference Products, Biosimilar Products, and Interchangeable Biosimilar Products
As biosimilars and interchangeable biosimilars become an integral part of modern biologic therapy, promotional communications around these products require heightened regulatory care. To address recurring industry questions and reduce the risk of misleading claims, the FDA has issued the guidance Promotional Labeling and Advertising Considerations for Prescription Biological Reference Products, Biosimilar Products, and Interchangeable Biosimilar Products . Published in 12 Dec

Sharan Murugan
Dec 13, 20254 min read


USFDA’s Draft Guidance: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product
The U.S. Food and Drug Administration (FDA) has released a new draft guidance titled “ Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Updated Recommendations for Assessing the Need for Comparative Efficacy Studies ” (October 2025). This document represents a major shift in the FDA’s scientific and regulatory approach to biosimilar product development, particularly regarding when comparative efficacy studies (CES) may or may not be required

Sharan Murugan
Nov 1, 20252 min read


USFDA Guidances: Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment, Other Quality-Related Considerations & Classification Categories for Certain Supplements
In 08 September 2025, the U.S. Food and Drug Administration (FDA) issued two final guidances addressing critical aspects of biosimilar...

Sharan Murugan
Sep 9, 20252 min read

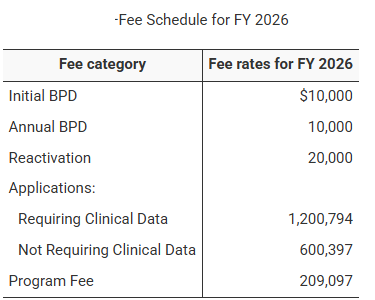
USFDA Announcement: Biosimilar User Fee Rates for Fiscal Year 2026 (October 1, 2025 – September 30, 2026)
On 30 July 2025, the U.S. Food and Drug Administration (FDA) officially published the Biosimilar User Fee Rates for Fiscal Year (FY) 2026...

Sharan Murugan
Aug 2, 20252 min read


MHRA’s Guidance: Access, New Active Substance and Biosimilar Work-Sharing Initiatives
In an era of rapid pharmaceutical innovation, global collaboration is key to expediting patient access to new therapies. To help patients...

Sharan Murugan
Jul 15, 20252 min read


UK MHRA: Guidance on the Licensing of Biosimilar Products
The Medicines and Healthcare products Regulatory Agency (MHRA) has updated its guidance on the " Licensing of Biosimilar Products " in...

Sharan Murugan
Dec 21, 20242 min read


USFDA Q&A Guidance: Postapproval Manufacturing Changes to Biosimilar and Interchangeable Biosimilar Products
Today (23 July,2024) the U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research, and Center for Biologics...

Sharan Murugan
Jul 22, 20242 min read


USFDA Guidance: Promotional Labeling and Advertising Considerations for Prescription Biological Reference and Biosimilar Products Q&A
On 24 April 2026 (Wednesday) the Center for Drug Evaluation and Research and the Center for Biologics Evaluation and Research released a...

Sharan Murugan
Apr 27, 20242 min read


Malaysia NPRA: Guidelines for Registration of Biosimilars in Malaysia
Malaysia's National Pharmaceutical Regulatory Agency (NPRA) last week (13 December 2023) released an updated guidance on "Guidelines for...

Sharan Murugan
Dec 19, 20231 min read


Swiss Medic: Biosimilar Authorisation Guidance
Swissmedic, the Swiss Agency for Therapeutic Products, published an updated guidance on (26 November 2023) the "Guidance document...

Sharan Murugan
Nov 26, 20232 min read


USFDA Guidance: Labeling for Biosimilar & Interchangeable Biosimilar Products
Earlier this Monday (18 September 2023), The U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research and Center...

Sharan Murugan
Sep 20, 20232 min read


USFDA Guidance: Assessing User Fees -BsUFA III: Biosimilar User Fee Amendments of 2022
Today (31 July 2023) the United States Food & Drug Administration's Center for Biologics Evaluation and Research and Center for Drug...

Sharan Murugan
Aug 1, 20232 min read


Switzerland's SwissMedic: Guidance on Authorisation Biosimilar
Yesterday (21 June 2023) Switzerland's Swissmedic released an updated guidance on "Authorisation Biosimilar" which specifies the...

Sharan Murugan
Jun 22, 20231 min read


MHRA Guidance: Licensing of Biosimilar Products
Recently (08-November-2022) the Medicines and Healthcare products Regulatory Agency (MHRA) released updated Guidance on the licensing of...

Sharan Murugan
Nov 12, 20221 min read


USFDA finalizes Q&As on Biosimilar Development
US Food and Drug Administration on 17th September, 2021 finalized additional questions and answers related to biosimilar development and...

Sharan Murugan
Sep 23, 20211 min read


Guidance on the Licensing of Biosimilar Products - United Kingdom (MHRA)
What is a Biosimilar Medicine? Biosimilar medicine is a biological medicine that is developed to be highly similar and clinically...

Sharan Murugan
May 8, 20211 min read
