USFDA Announcement: Biosimilar User Fee Rates for Fiscal Year 2026 (October 1, 2025 – September 30, 2026)
- Sharan Murugan

- Aug 2, 2025
- 2 min read
On 30 July 2025, the U.S. Food and Drug Administration (FDA) officially published the Biosimilar User Fee Rates for Fiscal Year (FY) 2026 in the Federal Register. These rates, effective from 1 October 2025 through 30 September 2026, fund FDA’s work in reviewing biosimilar product applications, helping ensure timely access to safe and effective biosimilar medicines in the United States.
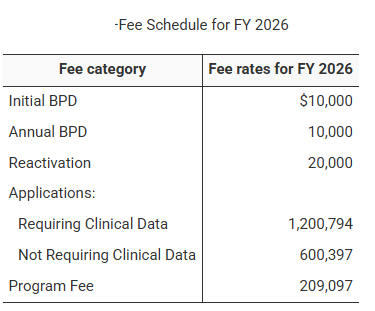
Under the Biosimilar User Fee Act (BsUFA), first passed in 2012 and most recently reauthorized as BsUFA III (Fiscal Years 2023–2027), the FDA collects fees from companies that develop biosimilar products.These fees help:
✅ Fund the review of biosimilar biologics license applications (BLAs)
✅ Support activities like product inspections, guidance development, and policy work
✅ Improve efficiency and predictability of the biosimilar review process
The BsUFA program is modelled on the successful PDUFA (for prescription drugs), but tailored to the scientific and regulatory complexity of biologics.
The fees are comprised of:
Biosimilar Biological Product Development (BPD) Fees: Includes initial, annual, and reactivation fees for participation in the FDA’s BPD program.
Application Fees: Paid upon the submission of biosimilar marketing applications, depending on whether clinical data is required for approval.
Program Fees: Collected annually from sponsors with approved biosimilar products.
New FY 2026 biosimilar user fee rates
For Fiscal Year 2026, sponsors should be aware of the following structures and key deadlines:
BPD Program Fees
Initial BPD Fee: Required when a sponsor first enters the BPD program.
Annual BPD Fee: Charged each subsequent year to remain in the program.
Reactivation Fee: For sponsors wishing to resume participation after withdrawal, equal to twice the annual BPD fee.
Biosimilar Application Fees
With clinical data required: A full application fee applies.
Without clinical data required: A 50% application fee is charged.
Annual Program Fees
Assessed per approved biosimilar product as of October 1 each year, with a cap of five per applicant.
These rates reflect adjustments for inflation, workload changes, and updated program costs. Payment timelines are strict—annual BPD and program fee invoices are sent in August, with payments due the first business day on or after October. Delays or missing payments may result in additional regulatory complications or delays in application review.
The BPD fees cover early-stage meetings with FDA to guide product development.
The BLA filing fee applies when submitting an application to license a biosimilar product for marketing.
The reactivation fee helps offset the cost when a sponsor restarts after exiting the BPD program.
Paying these fees allows sponsors to access FDA’s dedicated biosimilar review resources — a key factor in planning development timelines and budgets.
Staying Compliant: What Sponsors Need to Know
Discontinue BPD Program Participation: Sponsors must notify the FDA by August 1, 2025, to avoid being invoiced for the FY 2026 annual BPD fee.
Fee Payment Methods: Fees must be paid via Pay.gov (ACH), wire transfer, or by check/money order (never sent directly with submissions). Sponsors should reference their unique user fee ID.
For detailed numbers and instructions—including exact dollar amounts, fee reductions, exceptions, and payment processes—refer to the official Federal Register Notice.



Comments