Search


USFDA Med Dev Guidance: In Vitro Diagnostic Tests During Declared Emergencies
The U.S. Food and Drug Administration (FDA) has finalized a pivotal guidance addressing how the agency will decide when to begin and end...

Sharan Murugan
Oct 5, 20253 min read


USFDA Guidance: Software Assurance, Gene Therapies, and Clinical Designs
On 25 September 2025 , the U.S. Food and Drug Administration (FDA) highlighted four critical updated or reaffirmed guidances spanning...

Sharan Murugan
Oct 5, 20253 min read


USFDA Draft Guidance on Safety Labeling Changes: Streamlining Section 505(o)(4) Implementation
On September 19, 2025, the U.S. Food and Drug Administration (FDA) released its revised draft guidance, “ Safety Labeling...

Sharan Murugan
Sep 21, 20253 min read

USFDA Guidance: GERD-Related Drug Development: A Comprehensive Update
The U.S. Food and Drug Administration (FDA) released three important draft guidances on September 11, 2025, providing updated...

Sharan Murugan
Sep 21, 20252 min read


USFDA Draft Guidance: Development of Non-Opioid Analgesics for Chronic Pain
The U.S. Food and Drug Administration (FDA) has released a new draft guidance " Development of Non-Opioid Analgesics for Chronic Pain "...

Sharan Murugan
Sep 14, 20252 min read


USFDA Guidance: Alternative Tools for Facility Assessments in Pending Applications
The U.S. Food and Drug Administration (FDA) has issued a final guidance on 11th September 2025 titled “ Alternative Tools: Assessing Drug...

Sharan Murugan
Sep 14, 20252 min read


USFDA Guidances: Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment, Other Quality-Related Considerations & Classification Categories for Certain Supplements
In 08 September 2025, the U.S. Food and Drug Administration (FDA) issued two final guidances addressing critical aspects of biosimilar...

Sharan Murugan
Sep 9, 20252 min read


USFDA Med Dev Guidance: Animal Studies for Dental Bone Grafting Material Devices in 510(k) Submissions
On 22 August 2025 , the U.S. Food and Drug Administration (FDA) released a comprehensive guidance document titled “ Animal Studies for...

Sharan Murugan
Aug 23, 20252 min read


USFDA Draft Guidance: Radiopharmaceutical Dosage Optimization and Overall Survival Assesment in Oncology Clinical Trials
On 18th August 2025, the U.S. Food and Drug Administration (FDA) recently published two important draft guidance documents that aim to...

Sharan Murugan
Aug 19, 20252 min read


USFDA Guidance: Predetermined Change Control Plans (PCCPs) for AI/ML Medical Devices
Artificial Intelligence and Machine Learning (AI/ML) are increasingly used in medical devices—from diagnostic imaging software to digital...

Sharan Murugan
Aug 19, 20252 min read

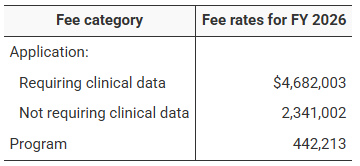
USFDA FDA User Fee Rates for FY 2026: A Comparative Overview for Prescription Drugs, Medical Devices, Generics, and Outsourcing Facilities
The U.S. Food and Drug Administration (FDA) has announced On 30 July 2025 , the official user fee rates across its major healthcare...

Sharan Murugan
Aug 2, 20252 min read

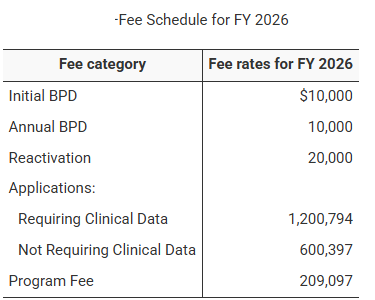
USFDA Announcement: Biosimilar User Fee Rates for Fiscal Year 2026 (October 1, 2025 – September 30, 2026)
On 30 July 2025, the U.S. Food and Drug Administration (FDA) officially published the Biosimilar User Fee Rates for Fiscal Year (FY) 2026...

Sharan Murugan
Aug 2, 20252 min read


USFDA Med Dev Guidance: Medical Device User Fee Small Business Qualification and Determination
In July 2025, the USFDA published a revised guidance titled " Medical Device User Fee Small Business Qualification and Determination "...

Sharan Murugan
Jul 31, 20252 min read

ICH -E21 (USFDA) Guidance: Including Pregnant and Breastfeeding Women in Clinical Trials
In May 2025, the FDA, as part of the International Council for Harmonisation (ICH), endorsed and released for consultation the draft E21...

Sharan Murugan
Jul 27, 20253 min read


USFDA Guidance: Formal Meetings Between the FDA and Sponsors or Applicants of BsUFA Products
In July 2025, the U.S. Food and Drug Administration (FDA) published its updated guidance for industry titled “ Formal Meetings Between...

Sharan Murugan
Jul 19, 20253 min read


USFDA Guidance: Drug Development for Myelodysplastic Syndromes (MDS)
Myelodysplastic syndromes (MDS) are a group of complex, clonal blood disorders that predominantly affect older adults and may progress to...

Sharan Murugan
Jul 5, 20252 min read


USFDA’s Draft Guidance: Aluminum Content and Labeling for Small Volume Parenteral Drug Products in Parenteral Nutrition
Aluminum contamination in parenteral nutrition (PN) has long posed a silent but serious risk—especially to vulnerable populations like...

Sharan Murugan
Jul 2, 20253 min read


ICH & USFDA Draft Guidance: Q1 Stability Testing: The Gold Standard for Drug Shelf Life and Quality
The stability of drug substances and drug products is a cornerstone of pharmaceutical quality, ensuring that medicines remain safe,...

Sharan Murugan
Jun 23, 20253 min read


USFDA Guidance: Post-Warning Letter Meetings Under GDUFA III: A Regulatory Pathway Toward Compliance
In the evolving regulatory landscape of generic drug manufacturing, compliance and transparency are more crucial than ever. The FDA’s...

Sharan Murugan
Jun 18, 20252 min read


USFDA Guidance: ANDAs: Pre-Submission Facility Correspondence Related to Prioritized Generic Drug Submissions
The U.S. Food and Drug Administration (FDA) has released an updated guidance " ANDAs: Pre-Submission Facility Correspondence Related to...

Sharan Murugan
Jun 13, 20252 min read
