Search

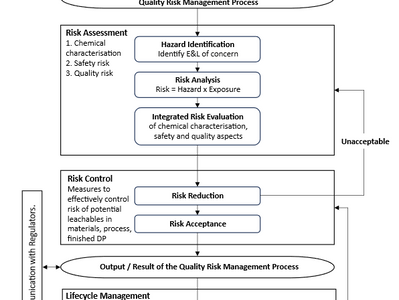
ICH Q3E – Impurities: Extractables and Leachables for Pharmaceuticals and Biologics
On 1 August 2025, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has...

Sharan Murugan
Aug 13, 20252 min read

ICH -E21 (USFDA) Guidance: Including Pregnant and Breastfeeding Women in Clinical Trials
In May 2025, the FDA, as part of the International Council for Harmonisation (ICH), endorsed and released for consultation the draft E21...

Sharan Murugan
Jul 27, 20253 min read

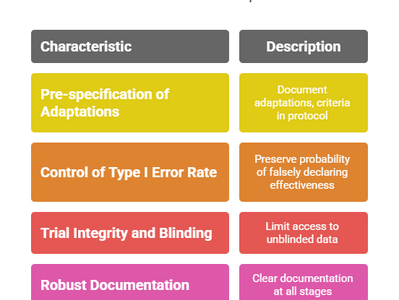
ICH E20 Draft Guideline: Understanding Adaptive Clinical Trials in Focus (2025)
The International Council for Harmonisation (ICH) has taken a major step toward modernizing clinical trial designs with the release of the...

Sharan Murugan
Jun 28, 20253 min read


ICH & USFDA Draft Guidance: Q1 Stability Testing: The Gold Standard for Drug Shelf Life and Quality
The stability of drug substances and drug products is a cornerstone of pharmaceutical quality, ensuring that medicines remain safe,...

Sharan Murugan
Jun 23, 20253 min read


ICH Guidance Q1: Stability Testing of Drug Substances and Drug Products (Step 2 Draft 2025)- Public Consultation
The International Council for Harmonisation (ICH) has released a significant update to its stability testing guidelines, consolidating...

Sharan Murugan
Apr 18, 20252 min read


Latest ICH Guidance updates: M15-Model-Informed Drug Development, E6(R3)-Good Clinical Practice: Annex 2, & E11A -Pediatric Extrapolation
The International Council for Harmonisation (ICH) continues to advance global standards in drug development and clinical practices with...

Sharan Murugan
Dec 28, 20242 min read


ICH Seeks Market Consultation for Global Post-Approval CMC Assessment Cloud Solution
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has issued a Request for...

Sharan Murugan
Nov 30, 20242 min read


FDA (ICH) Guidance: M12 Drug Interaction Studies & Questions and Answers
Yesterday (02 August, 2024), the Food and Drug Administration (FDA) announced that the final guidance on M12 drug interactions was...

Sharan Murugan
Aug 3, 20242 min read


ICH Guidance: M14 General Principles on Plan, Design, and Analysis of Pharmacoepidemiological Studies That Utilize Real-World Data for Safety Assessment of Medicines
FDA (FDA or Agency) has announced the availability of a draft guidance for the industry called “M14 General Principles on Planning,...

Sharan Murugan
Jul 3, 20242 min read


ICH M14 Guideline: General Principles on Plan, Design and Analysis of Pharmacoepidemiological Studies That Utilize Real-World Data for Safety Assessment of Medicines
Recently the International Council for Harmonisation (ICH) announced on 24 May, 2024 the availability of draft guidance for the industry...

Sharan Murugan
May 31, 20242 min read


ICH (Final) Guidance: Q2(R2) Validation of Analytical Procedures and Q14 Analytical Procedure Development
Last week the U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research, Office of Regulatory Policy, and the...

Sharan Murugan
Mar 13, 20241 min read


USFDA Guidance: ICH M7(R2) Assessment and Control of DNA Reactive (Mutagenic) Impurities
Earlier today (25 June, 2023) the United States Food & Drug Administration's Center for Drug Evaluation and Research, and Center for...

Sharan Murugan
Jul 24, 20232 min read


USFDA/ICH - E6(R3) GCP Guideline: Modernizing the Design & Conduct of Clinical Trials
The US Food and Drug Administration has announced earlier today (06 June, 2023) the availability of a draft guidance "E6(R3) Good...

Sharan Murugan
Jun 6, 20231 min read


ICH/USFDA Final Guidance: Q9(R1) Quality Risk Management
Yesterday (03 May, 2023) the United States Food & Drug Administration's Center for Drug Evaluation and Research and Center for Biologics...

Sharan Murugan
May 4, 20231 min read


ICH/ USFDA Guidance: S12 Nonclinical Biodistribution Considerations for Gene Therapy Products
On 27th April, 2023 the United States Food & Drug Administration Center for Drug Evaluation and Research, and Center for Biologics...

Sharan Murugan
Apr 29, 20232 min read


ICH-Quality Guidance: Q13 Continuous Manufacturing of Drug Substances and Drug Products
In today's FDA press release, the ICH final guidance titled "Q13 Continuous Manufacturing of Drug Substances and Drug Products" was...

Sharan Murugan
Mar 1, 20231 min read


ICH (USFDA) Guidance: Bioequivalence for Immediate-Release Solid Oral Dosage Forms
The USFDA released draft guidance for the industry on January 31, 2023, “M13A Bioequivalence for Immediate-Release Solid Oral Dosage...

Sharan Murugan
Feb 1, 20231 min read


ICH M11 Guidance: Tech Spec - Clinical Electronic Structured Harmonised Protocol (CESHARP)
Recently on 21-December-2022, U.S Food and Drug Administration announced the availability of a draft guidance for industry entitled “M11...

Sharan Murugan
Dec 27, 20222 min read


ICH Guidance: Q3D(R2) ELEMENTAL IMPURITIES
Recently the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) released the...

Sharan Murugan
Sep 17, 20221 min read


ICH Guidances: Q2(R2) Validation of Analytical Procedures, Q14, M12, E11A, E14 & S7B Updation
In an announcement yesterday, USFDA released the below draft guidances for industry, developed with the assistance of ICH, the...

Sharan Murugan
Aug 27, 20222 min read
